Non-communicable diseases (NCDs) account for 63% of the worldwide mortality [1 ]. Importantly, 80% of these deaths occur in low and middle-income countries (LMIC) [1,2]. Evidence-based interventions to prevent and manage NCDs in LMIC are urgently needed to face their projected rising incidence [1]. Yet, research on the epidemiology, determinants, care delivery aspects, and outcomes of NCDs in low-resource settings is disproportionately scarce. NCDs in pregnancy include pre-existing and gestational diabetes mellitus (DM) as well as hypertensive disorders of pregnancy (HDP). These NCDs are associated with adverse maternal, pregnancy, and perinatal outcomes [3,4] and carry long-term risks of cardiovascular morbidity for both mothers and their offspring [5-7]. As a result, developing and evaluating setting-adapted screening and management programs for NCDs in pregnancy are among the World Health Organization’s (WHO) research priorities [8].
The maternal mortality ratio in Haiti is estimated at 359 per 100 000 live births, representing the highest ratio in the Western hemisphere [9]. Neonatal mortality at 28 per 1000 live births remains one of the highest in the world [10]. Despite this high maternal-neonatal mortality, information on national prevalence of NCDs in pregnancy and their impact on maternal and neonatal outcomes is lacking. Indeed, while most women in Haiti are screened at least once for HDP during their antepartum follow-up [11], a national screening and treatment program for gestational DM has not yet been implemented.
We conducted the “Diabete et hYpertension Arterielle et leurs issues MAternelles et Neonatales” (DYAMAN) study, a collaborative research initiative between Haitian and Canadian health care providers and investigators aiming to contribute further evidence on the prevalence of NCDs in pregnancy and their associated perinatal outcomes in a regional hospital in Haiti.
Ethics considerations
Written informed consent for participation was obtained after explaining aims, format, and risks of the study to eligible women in either Haitian Creole or French language, according to patient preference. For patients not able to read, the consent form was read aloud. Patients unable to write signed by marking a cross in the presence of a witness who co-signed the consent form. A study coordinator fluent in both languages was available for questions during the consent process.
Study population
We conducted a prospective cohort study at the Saint-Nicolas Hospital, in the community of Saint-Marc, in the Artibonite region of the republic of Haiti between June 1, 2017 and February 28, 2019. Women presenting to routine care at the hospital’s antenatal clinic between 24 and 28 weeks of gestation were eligible. Potential participants were informed about the study during their scheduled appointment with a midwife and were then approached by study personnel. Women with a pregnancy of less than 24 weeks of gestational age and women who were not fasting yet interested in participating in the study were given the opportunity to enroll later. Women <18 years of age and those who were not pregnant were excluded.
At time of cohort entry, research personnel fluent in French and Haitian Creole administered an initial survey. This survey was comprised of demographic information (including age, education, profession, marital status, neighborhood of residence, living situation), past obstetrical history (including date of last menstruation period, current gestational age, number of prior pregnancies and prior abortions, prior history of fetal demise, preterm delivery, small for gestational age baby, or hypertensive disorders of pregnancy and gestational diabetes), past medical history (including known hypertensive disorder, type 1 or type 2 diabetes, history of cerebrovascular accident, history of anemia, and known HIV status), family history (including maternal history of hypertensive disorder of pregnancy, diabetes, or cardiac history), and social history (including number of daily meals, and history of tobacco smoking and alcohol consumption). Anthropometric measurements were also recorded (including weight, height, and fundal height measured by obstetric midwives with metric-based measurement tape); body mass index (BMI) was calculated.
Screening for gestational diabetes and hypertensive disorders of pregnancy
A one-step oral glucose tolerance test (oGTT) for the diagnosis of gestational diabetes mellitus was performed using a 75 g oral glucose solution (Glucose-75, JAMP, Boucherville, Canada) [12]. This consisted in 300 mL of oral beverage. We measured glycemia at time 0, 1h, and 2h after drinking the oral glucose solution with a high-precision, high-accuracy portable glucometer (Countour Next, Ascensia Diabetes Care, NJ, USA) [13,14]. We opted for glucometer-based capillary glucose measurements as laboratory-based serum glucose analysis is seldom available at the regional hospital (as is the case for most public health care facilities in Haiti) [14,15]; an approach that has previously been shown to be accurate in settings were laboratory glucose measurements are not reliably available [16]. Women with fasting glycemia >92 mg/dL (5.1 mmol/L), 1h glycemia >180 mg/dL (10 mmol/L), or 2h glycemia >153 mg/dL (8.5 mmol/L) were considered as having gestational DM [12]. Women with preexisting diabetes or women with fasting glycemia >180 mg/dL did not undergo further oGTT and were considered as having pre-existing DM.
Blood pressure (BP) measurements were taken twice at rest, 15 minutes apart, and averaged using an electronic sphygmomanometer (OMRON M7, OMRON, Kyoto, Japan) validated for use in pregnancy [17]. Urinary dipstick testing assessed proteinuria. Hypertensive disorders included chronic hypertension, gestational hypertension, and preeclampsia. Women were considered as having chronic hypertension if they reported a diagnosis of hypertension or were treated for hypertension <20 weeks of gestation. They were considered as having gestational hypertension if they had persistent systolic BP≥140 mm Hg or a diastolic BP≥90 mm Hg at ≥20 weeks of gestation [18]. Preeclampsia was diagnosed when chronic or gestational hypertension was accompanied by new onset proteinuria, pulmonary edema, liver enzyme abnormalities, thrombocytopenia, neurologic symptoms, intractable hypertension, or fetal distress [4,18]. Women without DM and without HDP were considered as the control group.
Antepartum management
Follow-up visit frequency was determined by study group (Appendix S1 in the Online Supplementary Document ). Women could change groups during follow-up if they had a new NCD diagnosis.
Clinical protocols for the management of DM were co-developed by Haitian and Canadian investigators adapted from international society guidelines from the International Federation of Gynecology and Obstetrics (FIGO), the Canadian Diabetes Association (CDA), and the National Institute for Health and Care Excellence (NICE) [5, 15,19]. Women with DM received nutrition and exercise counseling by a specialized diabetes nurse. They were given portable glucometers (Contour Next, Ascensia Diabetes Care, NJ, USA) for home glucose monitoring. Glycemic targets were 80-90 mg/dL (4.4-5.0 mmol/L) fasting and 110-140 mg/dL (6.1-7.8 mmol/L) 1 hour after a meal. Participants with fasting glycemia <92 mg/dL (5.1 mmol/L) were treated with non-pharmacologic measures only; those with fasting glycemia 92-126 mg/dL (5.1 and 7.0 mmol/L) were also treated with metformin, with a targeted dose of 1g twice daily; those with fasting glycemia >126 mg/dL (7.0 mmol/L) required both metformin and nightly subcutaneous insulin NPH (Appendix S2 in the Online Supplementary Document ). If the postprandial glycemia was >150 mg/dL twice in a week, insulin R was given with meals. Women were followed at least weekly initially (Appendix S1 in the Online Supplementary Document ).
As per local protocols already in place at the beginning of the study conduct, women with BP≥160/110 mm Hg were admitted for inpatient monitoring and management of severe hypertension. Systolic BP targets were 130-155 mm Hg and diastolic BP targets were 80-105 mm Hg. Medications locally available for use included intravenous labetalol and hydralazine, short acting nifedipine, as well as oral methyldopa, labetalol, and long acting nifedipine.
Peripartum management
Women with DM were managed according to a local intrapartum protocol using subcutaneous insulin R every 2h (Appendix S3 in the Online Supplementary Document ). Women with HDP had frequent BP monitoring, and were given intravenous or intramuscular magnesium sulfate, if indicated (Figure S4 in the Online Supplementary Document ). Information was collected on delivery mode, obstetrical complications, glycemia, BP measurements, neonatal birth weight, and neonatal complications. Fetal and maternal deaths were also recorded.
Neonatal management
The pediatric team assessed infants of mothers with DM, and those of mothers with HDP if they were born with low birth weight (birth weight <2500 g), macrosomia (birth weight >4000 g), or if they were born <37 weeks of gestation. Small for gestational age (SGA) was defined as birth weight lower than the 10th percentile for gestational age. First line therapy for neonatal hypoglycemia was maternal breastfeeding or formula milk followed by either continued oral nutrition or intravenous dextrose according to severity of hypoglycemia (Appendix S4 in the Online Supplementary Document ). Neonatal deaths were recorded.
Data collection
Outpatient data was prospectively collected by the study nurse at every outpatient study visit. Inpatient data was retrospectively collected from a detailed medical chart review. Study data were collected and managed using REDCap electronic data capture tools hosted at the Research Institute of the McGill University Health Centre [20,21].
Statistical analysis
Clinical characteristics were described using standard descriptive measures. Analyses were performed across predefined diagnosis groups (ie, control, DM, HDP, DM/HDP groups). Comparisons between continuous and categorical variables were made with analysis of variance (ANOVA) and Fisher’s exact test, respectively. Assuming prevalence estimates of gestational diabetes between 10 and 15%, at a precision of 5%. and with a 95% confidence interval, we estimated that a sample size of 657 women was required given an oGTT’s sensitivity and specificity of 0.7 and 0.9. Oversampling of 5%-10% was foreseen to account for loss to follow-up. A P value ≤0.05 was considered statistically significant. All statistical analyses were performed with RStudio (version 1.1.383, Boston, MA, USA).
Study population and baseline characteristics
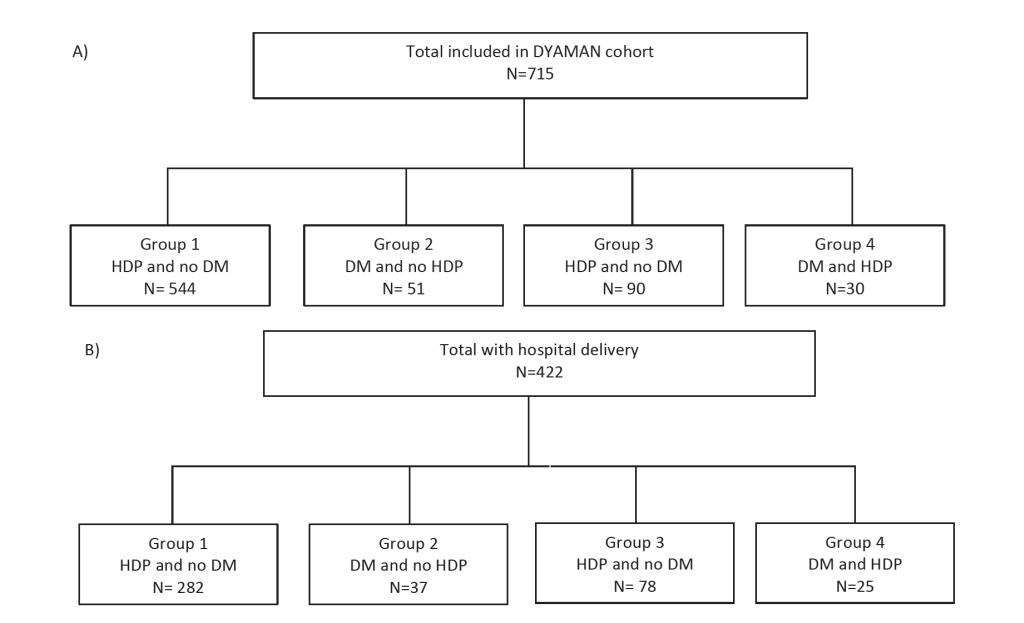
In total, 715 women aged 29 years on average, were included in the DYAMAN cohort, comprising 544 (76.1%) controls, 51 (7.1%) women with DM only, 90 (12.6%) women with HDP only, and 30 (4.2%) women with DM/HDP ( Table 1 and Figure 1 , Panel A). In total, DM affected 81 (11.3%) women and HDP affected 120 (16.8%) women. Women with DM were more likely to be of maternal age ≥35 years than controls, and women with HDP ( Table 1 ). Women with HDP had higher rates of obesity than controls, whereas women with DM did not ( Table 1 ). Alcohol use was more common among women with than without DM. Moreover, women with DM were more likely to have 0 years of education than women without DM. Although most women lived in houses built with concrete, at least a quarter of them were affected by food insecurity ( Table 1 ). Missing values for variable included in Table 2 can be found on Table S1 in the Online Supplementary Document .
| Total, n = 715 | Control, (N = 544) | DM, (N = 51) | HDP, (N = 90) | DM/HDP, (N = 30) | P-value |
|---|---|---|---|---|---|
| Gestational age at initial visit, weeks, mean ± SD | 26.5 ± 1.6 | 26.3 ± 1.8 | 26.7 ± 1.5 | 26.1 ± 1.5 | 0.405 |
| Age, mean ± SD | 28.2 ± 6.1 | 30.8 ± 7.5 | 29.8 ± 5.5 | 32.1 ± 6.2 | <0.0001 |
| Maternal age ≥35 years, n (%) | 79 (14.5) | 14 (27.5) | 14 (15.9) | 10 (33.3) | 0.011 |
| BMI, mean ± SD | 26.3 ± 4.9 | 26.9 ± 5.2 | 27.7 ± 5.8 | 29.4 ± 6.3 | 0.002 |
| -Underweight (BMI <18.5), n (%) | 6 (1.1) | 2 (3.9) | 1 (1.1) | 1 (3.3) | 0.164 |
| -Obesity (BMI ≥30), n (%) | 109 (20) | 8 (15.7) | 31 (34.4) | 13 (43.3) | <0.001 |
| Prior pregnancies, number, mean ± SD | 1.3 ± 1.4 | 1.5 ± 1.6 | 1.4 ± 1.4 | 2.2 ± 2.0 | 0.008 |
| Live children, number, mean ± SD | 1.6 ± 1.2 | 1.6 ± 1.6 | 1.5 ± 1.1 | 1.9 ± 1.9 | 0.646 |
| Matrimonial status: | |||||
| Married, n (%) | 196 (36) | 23 (45.1) | 35 (38.9) | 10 (33.3) | 0.580 |
| Living with partner, n (%) | 174 (32) | 16 (31.4) | 32 (35.6) | 14 (46.7) | 0.376 |
| Single, n (%) | 173 (32) | 12 (23.5) | 23 (25.6) | 6 (20) | 0.269 |
| Education length, year, mean ± SD | 11.0 ± 3.7 | 10.4 ± 4.5 | 11.1 ± 3.6 | 9.7 ± 4.5 | |
| 0 years of education, n (%) | 11 (2) | 4 (7.8) | 2 (2.2) | 2 (6.7) | 0.036 |
| <10 years of education, n (%) | 142 (26.1) | 11 (21.6) | 21 (23.3) | 11 (36.7) | 0.480 |
| House built with concrete, n (%) | 475 (87.3) | 45 (88.2) | 80 (88.9) | 29 (96.7) | 0.063 |
| Food insecurity, n (%) | 147 (27) | 14 (27.5) | 23 (25.6) | 9 (30) | 0.960 |
| Current smoker, n (%) | 3 (0.6) | 0 | 0 | 0 | 1 |
| Current alcohol use, n (%) | 19 (3.5) | 5 (9.8) | 6 (6.7) | 1 (3.3) | 0.099 |
| Comorbidities: | |||||
| One elevated blood pressure in lifetime, n (%) | 32 (5.9) | 1 (2.0) | 21 (23.3) | 11 (36.7) | <0.001 |
| -Preexisting diabetes, n (%) | 1 (0.2) | 7 (13.7) | 0 | 5 (16.7) | <0.001 |
| Obstetrical history:* | |||||
| Gestational hypertension, n (%) | 37 (11.8) | 3 (10.0) | 23 (40.4) | 6 (30.0) | <0.001 |
| Preeclampsia, n (%) | 12 (3.8) | 1 (3.3) | 11 (19.3) | 2 (10.0) | 0.001 |
| Prior testing for gestational diabetes, n (%) | 32 (10.2) | 3 (10.0) | 6 (10.5) | 2 (10.0) | 1.000 |
BMI – body mass index, DM – diabetes Mellitus, HDP – hypertensive disorder of pregnancy, SD – standard deviation
*Obstetrical history was obtained from women who had delivered at least once.
| Attendance | Initial visit (n = 715) | Visit 2 (n = 604) | Visit 3 (n = 489) | Visit 4 (n = 313) | Visit 5 (n = 144) | Visit 6 (n = 63) | Delivery (n = 422) |
|---|---|---|---|---|---|---|---|
| Gestational age, week, median (IQR) | 26.7 (25.1-28.0) | 31 (29.6-32.6) | 34.7 (32.9-36.1) | 36.9 (35.0-38.3) | 38 (36-39.4) | 38.4 (35.3-40) | 39 (37.8-40.2) |
| Control, n (%) | |||||||
| -DM, n (%) | 71 (9.9) | 65 (10.8) | 58 (11.9) | 53 (16.9) | 44 (30.6) | 33 (52.4) | 42 (10.0) |
| -HDP, n (%) | 29 (4.1) | 21 (3.5) | 19 (3.9) | 22 (7.0) | 9 (6.3) | 3 (4.8) | 60 (14.2) |
| -DM/HDP, n (%) | 10 (1.4) | 10 (1.6) | 10 (2.0) | 9 (2.9) | 6 (4.2) | 5 (7.9) | 20 (4.7) |
| Total patients with DM (N = n present/n expected) | N = 81/81 | N = 75/81 | N = 68/81 | N = 62/81 | N = 50/81 | N = 38/81 | N = 62/81 |
| -Minimum fasting BG, mg/dL, median (IQR) | – | 74(66-84) | 76 (70-83) | 73 (69-81) | 76 (71-81) | 73 (68-79) | – |
| -Maximum fasting BG, mg/dL, median (IQR) | – | 110 (93-135) | 106 (95-131) | 105 (93-128) | 97 (88 -117) | 102 (90-116) | – |
| -Minimum after 1h, mg/dL, median (IQR) | – | 91 (78-101) | 85 (73-99) | 84 (74-95) | 84 (77-99) | 90 (77-96) | – |
| -Maximum after 1h, mg/dL, median (IQR) | – | 150 (127-173) | 142 (126-182) | 150 (128-179) | 154 (124-172) | 150 (124-184) | – |
| -Diet only, n (%)* | N/A | 43 (57.3) | 42 (61.8) | 37 (59.7) | 29 (58.0) | 20 (52.6) | – |
| -Metformin, n (%)* | 11 (13.6) | 21 (28.0) | 20 (29.4) | 14 (22.6) | 14 (28.0) | 12 (31.6) | – |
| -Insulin, n (%)* | 1 (1.2) | 1 (1.3) | 1 (1.5) | 1 (1.6) | 1 (2.0) | 0 | – |
| -Metformin and insulin, n (%)* | 0 | 2 (2.7) | 3 (4.4) | 6 (9.7) | 4 (8.0) | 4 (10.5) | – |
| -Metformin changed to insulin, n -(%)* | N/A | 2 (2.7) | 1 (1.5) | 3 (4.8) | 0 | 0 | – |
| -Daily dose of metformin (mg/d) | 473 ± 75 | 413 ± 122 | 448 ± 144 | 453 ± 98 | 486 ± 160 | 469 ± 125 | – |
| -Daily dose of insulin (Units/d) | 8 ± 4 | 6 ± 1 | 8 ± 3 | 8 ± 3 | 8 ± 3 | 6 ± 2 | – |
| Total patients with HDP (N = n present/n expected) | N = 39/39 | N = 31/50 | N = 29/59 | N = 31/68 | N = 15/68 | N = 8/69 | N = 103/120 |
| -Systolic blood pressure, mean ± SD | 115 ± 17 | 115 ± 17 | 118 ± 16 | 121 ± 17 | 120 ± 15 | 118 ± 17 | 143.4 ± 19.2 |
| -Diastolic blood pressure, mean ± SD | 72 ± 13 | 72 ± 13 | 75 ± 13 | 78 ± 13 | 79 ± 13 | 77 ± 11 | 90.0 ± 15.8 |
| -Monotherapy, n (%):† | 27 (84.4) | 18 (58.1) | 20 (69.0) | 16 (51.6) | 10 (66.7) | 4 (50.0) | – |
| Methyldopa, n (%)† | 18 (56.3) | 15 (48.3) | 16 (55.2) | 15 (48.4) | 10 (66.7) | 4 (50.0) | – |
| Nifedipine, n (%)† | 0 | 0 | 0 | 0 | 0 | 0 | – |
| Other, n (%)† | 8 (25.0) | 3 (9.7) | 4 (13.8) | 1 (3.2) | 0 | 0 | – |
| -Dual therapy, n (%)† | 1 (3.1) | 0 | 0 | 0 | 0 | 0 | – |
| Preeclampsia, n (%) | – | – | – | – | – | – | 58 (13.7) |
*Denominator composed of women with DM and DM/HDP present at clinic visit.
†Denominator composed of women with HDP and DM/HDP.
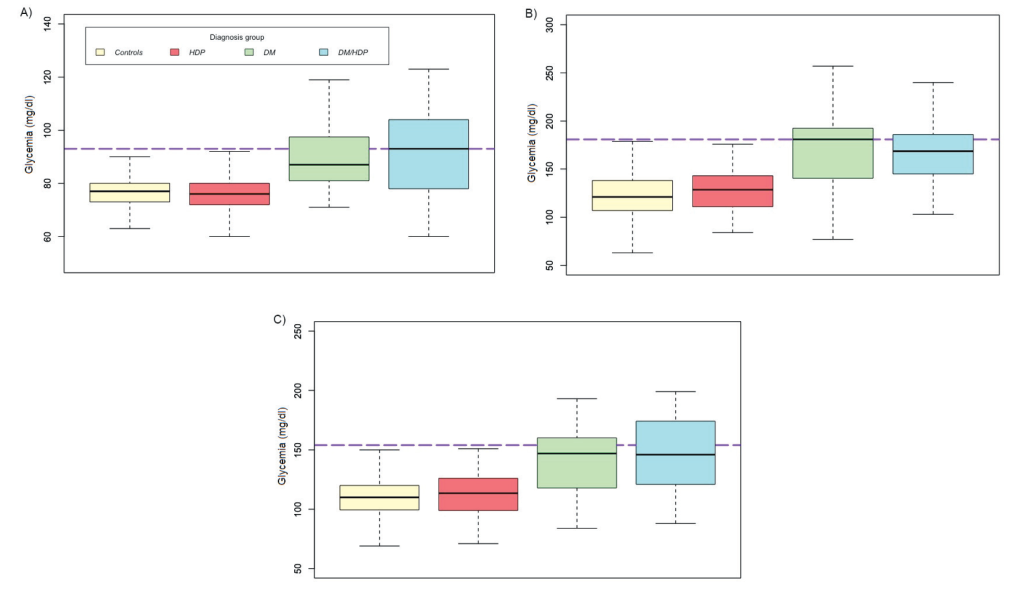
At time of diagnostic testing for DM, women with DM/HDP tended to have higher fasting glycemia than women with DM alone (median DM = 87 mg/dl vs DM/HDP = 93 mg/dl, P < 0.41, Figure 2 , Panel A), whereas women with DM tended to have higher postprandial glycemia at 1h (median DM = 181 mg/dl vs DM/HDP = 168.5 mg/dl, P < 0.824, Figure 2 , Panel B). No differences were found among women with HDP and controls at 1h (median HDP = 128.5 mg/dl vs control = 121 mg/dl, P = 0.056) and 2h (median HDP = 113.5 mg/dl vs control = 110 mg/dl, P = 0.288, Figure 2 , Panels B and C).
Clinical parameters during antepartum follow-up
Attendance at the antepartum clinic ranged from 63 (8.8%) to 604 (84.5%) patients per visit ( Table 2 ). On average, women in the control group had 4.7 ± 1.8 of 6 foreseen visits, and women in the DM, HDP, and DM/HDP groups had 8.0 ± 2.5, 5.2 ± 2.1, and 8.2 ± 2.3 of a minimum of 12, 8 and 12 foreseen visits, respectively. Attrition was most pronounced on visits at 35-40 weeks of gestation.
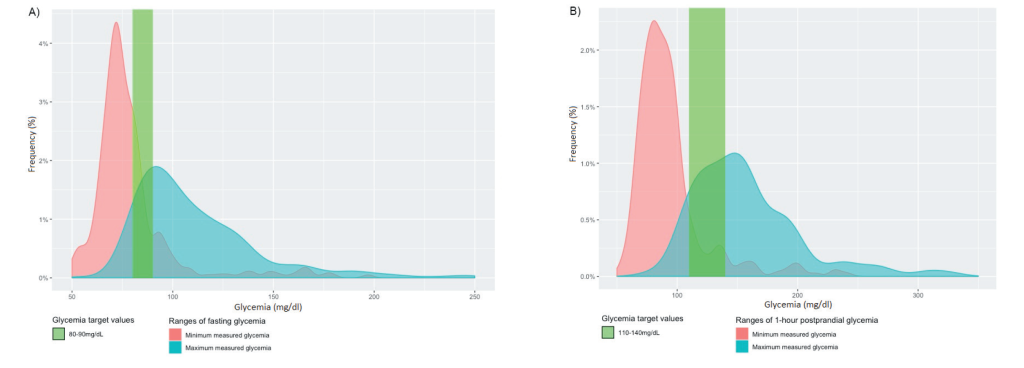
Among patients with DM, 39 (58.2%) did not receive pharmacotherapy, 21(31.3%) were on metformin only, 6 (9%) were on metformin and insulin, and 1 (1.5%) was on insulin only ( Table 2 ). Metformin was given at an average dose <500 mg/d ( Table 2 ). During follow-up, medians (IQR) for minimum and maximum fasting glycemia were 74 (68-81) and 102 (89-125) mg/dL (Figure 3A), and medians (IQR) for minimum and maximum 1-hour postprandial glycemia were 86 (76-99) and 150 (124-182) mg/dL, indicating an overall reasonable control for women who attended follow-up visits ( Figure 3 , Panel B). Most patients with HDP required pharmacologic monotherapy for BP ( Table 2 ). Mean systolic BP during follow-up ranged from 115.1 ± 16.9 to 120.8 ± 16.5 mm Hg and mean BP pressure ranged from 77.2 ± 12.5 to 79.0 ± 12.9 mm Hg ( Table 2 ).
Perinatal outcomes
A total of 422 (59%) women were delivered at the hospital: 282 (66.8%) controls, 37 (8.8%) with DM, 78 (18.5%) with HDP, and 25 (5.9%) with DM/HDP ( Figure 1 , Panel B). The mean gestational age was highest among controls and lowest among women with HDP (Table 3). Preterm birth <32 weeks was more common among women the HDP group than among the control, DM, and DM/HDP groups ( Table 3 ). Vaginal deliveries occurred more commonly in controls than in women with DM and/or HDP ( Table 3 ).
| Total (N = 422) | Controls (N = 282) | DM (N = 37) | HDP (N = 78) | DM/HDP (N = 25) | P-value* |
|---|---|---|---|---|---|
| Gestational age at delivery, weeks, mean ± SD (n = 168) | 39 ± 2.4 | 39.4 ± 3.0 | 37.9 ± 3.9 | 38.7 ± 2.1 | 0.893 |
| <32weeks, n (%) | 1 (0.4) | 0 | 4 (5.1) | 0 | 0.023 |
| 32-36 weeks, n (%) | 14 (5.0) | 3 (8.1) | 6 (7.7) | 1 (4.0) | 0.529 |
| ≥37 weeks, n (%) | 99 (35.1) | 10 (27.0) | 23 (29.5) | 7 (28.0) | 0.108 |
| Delivery mode: | |||||
| Vaginal, n (%) | 227 (80.4) | 21 (56.8) | 60 (76.9) | 16 (64.0) | <0.001 |
| Cesarean delivery, n (%) | 19 (6.7) | 10 (27.0) | 15 (19.2) | 5 (20.0) | <0.001 |
| Forceps, n (%) | 1 (0.4) | 0 | 0 | 0 | 1 |
| Maternal blood glycemia, mean ± SD (n = 3) | n/a | 72 | n/a | 72 ± 5 | 0.948 |
| Maternal blood pressure (n = 337): | |||||
| BP≥160/110 mm Hg, n (%) | n/a | n/a | 20 (25.6) | 5 (20.0) | 0.789 |
| Systolic BP, mmHg, mean ± SD | 116 ± 12 | 120 ± 11 | 144 ± 19 | 143 ± 21 | <0.001 |
| Diastolic BP, mmHg, mean ± SD | 70 ± 10 | 74 ± 10 | 91 ± 17 | 89 ± 13 | <0.001 |
| Preeclampsia, n (%) | 0 | 0 | 45 (57.7) | 13 (52.0) | <0.001 |
| Eclampsia, n (%) | 0 | 0 | 5 (6.4) | 0 | 0.002 |
| Small for gestational age, n (%) | 11 (3.9) | 0 | 3 (3.8) | 2 (8.0) | 0.320 |
| Large for gestational age, n (%) | 21 (7.4) | 6 (16.2) | 6 (7.7) | 1 (4.0) | 0.170 |
| Birth weight (n = 362): | |||||
| <2500 g, n (%) | 20 (7.1) | 1 (2.7) | 20 (25.6) | 3 (12.0) | <0.001 |
| 2,500-4000 g, n (%) | 212 (75.2) | 24 (64.9) | 53 (67.9) | 17 (68.0) | 0.004 |
| >4000 g, n (%) | 6 (2.1) | 5 (13.1) | 1 (1.3) | 0 | 0.008 |
| Obstetrical complications (n = 366) | |||||
| Shoulder dystocia, n (%) | 0 | 0 | 1 (1.3) | 0 | 0.339 |
| Perinatal asphyxia, n (%) | 0 | 0 | 0 | 0 | 1 |
| Neonatal hypoglycemia, n (%) (n = 64) | n/a | 1 (2.7) | 0 | 2 (8.0) | 0.366 |
| Intravenous dextrose, n (%) | – | 1 (100) | – | 1 (100) | 1 |
| Neonatal complications (n = 363): | |||||
| Fetal death, n (%) | 2 (0.7) | 1 (2.7) | 1 (1.3) | 2 (8.0) | 0.017 |
| Neonatal death, n (%) | 3 (1.1) | 0 | 3 (3.8) | 3 (12.0) | 0.013 |
BP – blood pressure, DM – diabetes mellitus, HDP – hypertensive disorders of pregnancy, SD – standard deviation
*P-value for difference between groups.
While 39 women had an HDP at the time of first visit, 30 women developed HDP during follow-up (n = 69 women at the end of antepartum follow-up), and 51 manifested with HDP at time of delivery (n = 120 by time of delivery, Table 2 ). Among women with HDP, systolic BP was above target upon presentation for delivery, with at least 20% presenting with severe hypertension ( Table 3 ). Preeclampsia affected 58 (13.7%) women (n = 45 in the HDP group, n = 15 in the DM/HDP group), which represented 56.3% of all HDP deliveries (Table 3). Five women in the HDP group had eclampsia and for three of them, eclamptic seizures were the first manifestation of HDP ( Table 3 ).
Babies with low birth weight (<2500 g) were more common in the HDP than in the control and DM groups ( Table 3 ). However, there was no difference in the number of babies born SGA ( Table 3 ). Macrosomia was more frequent among women with DM than in the control and HDP groups ( Table 3 ). Primarily due to a shortage of pediatric staff, protocol deviation for hypoglycemia screening in infants of diabetic mothers occurred in 19 such that only 43 of 62 neonates who would have had a blood glucose check at 1 hour of life were actually tested. Neonatal hypoglycemia was reported in three (7%) neonates of 43 mothers with DM and DM/HDP, two of which required intravenous dextrose ( Table 3 ). Perinatal mortality. designating fetal and neonatal deaths, affected 15 (36 per 1000) deliveries, including 5 (18 per 1000) deliveries in the control, 1 (27 per 1000) delivery in the DM, 4 (51 per 1000 deliveries) deliveries in the HDP, and 5 (200 per 1000) deliveries in the DM/HDP groups. Missing information for variables included in Table 3 can be found in Table S2 of the Online Supplementary Document .
We conducted a prospective cohort study of pregnant women with and without DM and HDP in in a regional hospital in Haiti. We reported on the local prevalence of these NCDs and their associated perinatal outcomes. We found that DM and HDP affected 11.3% and 16.8% of women in our cohort, respectively. While BP management was optimized during antepartum follow-up, systolic BP was above target upon presentation for delivery among women with HDP. Infants exposed to maternal DM had more macrosomia than those in the control, HDP, and DM/HDP groups. More early preterm births <32 weeks occurred among women with HDP, resulting in a higher proportion of infants with low birth weight in this group. The overall perinatal mortality rate in our cohort was mostly driven by deaths among infants of mothers with DM and/or HDP. This study will help to prepare future refinements to optimize the management of NCDs in pregnancy in order to reduce adverse maternal and neonatal outcomes.
Diabetes in pregnancy
In our cohort, obesity was associated with HDP, yet not with gestational DM. Risk factors for hyperglycemia may vary in a context of food insecurity and undernutrition [22], which further supports universal screening for gestational DM in LMIC, as traditional cardiometabolic risk factors may not apply. High rates of macrosomia and perinatal mortality among women with DM in our study suggests that antenatal hyperglycemia likely persisted for some women. High rates of macrosomia and perinatal mortality among women with DM in LMIC have also been reported by others [23-25]. While the median maximum self-measured glycemia of women who attended follow-up was 10 mg/dL above target, the median maximum glycemia of women who were not present at follow-up may have been higher, leading to an underestimation of suboptimal glycemic control in the cohort. Although we found that less neonates had macrosomia among women with DM/HDP than DM alone, a normal weight at birth may not have been synonymous with metabolic health. Indeed, the effects of hyperglycemia on fetal weight may have been offset by the presence of intrauterine growth restriction.
Hypertensive disorders of pregnancy
In Latin America and the Caribbean HDP account for 22.2% of all maternal deaths [26]. The incidence of preeclampsia among women delivered in hospital in our study was comparable to the incidence reported by others [27], which was almost three times higher than the estimated global incidence of ~ 5% [28]. This proportion should however be interpreted with caution since 41% of women in our cohort were not delivered in hospital. The high rates of preeclampsia in our study may warrant consideration for preventive measures such as universal prophylaxis with low-dose aspirin [29].
Between the last scheduled antenatal visit and delivery, 40 women from the control group developed HDP, preeclampsia being the most common diagnosis. Multifactorial delays in triage, transport, and treatment of women with HDP may explain the increased burden of adverse outcomes associated with HDP in LMIC [30]. Since most women in our cohort were diagnosed with preeclampsia at time of admission for delivery, a timelier diagnosis of preeclampsia could possibly improve maternal and neonatal outcomes. In addition, we observed a high incidence of eclampsia in our study, which may be decreased with tighter BP management at time of admission for delivery, and a low threshold to initiate magnesium sulfate prophylaxis. Community level interventions with facility enhancement have been suggested to decrease preeclampsia-related adverse outcomes [30,31].
Challenges in the implementation of care delivery algorithms
Our study illustrates some challenges in caring for NCDs in low-income countries [2]. To overcome limitations in laboratory equipment, we used portable glucometer for oGTT and point-of-care urinary dipstick tests for proteinuria assessment. The evaluation of women with preeclampsia was limited by unavailability of kidney and liver function tests, despite both being featured in the Second WHO Model List of Essential In Vitro Diagnostics [ 32]. We had to contend with supply shortages, as the provision of Glucola, portable glucometers, and strips, was intermittently interrupted during the study period. Shortages in insulin occurred despite it being on the WHO Model List of Essential Medicine [33]. We obtained additional insulin from neighbouring pharmacy and diabetes clinic when the insulin was unavailable. Human shortages were present at multiple steps of patient care. Intrapartum maternal and neonatal glucose monitoring protocols were particularly challenging. Such barriers may represent important hurdles to overcome in order to insure optimal NCD care in LMIC.
Strengths and limitations
Our study had several strengths. We introduced an innovative clinical care delivery program for DM in pregnancy using home glucose monitoring, oral agents, and insulin in a public hospital in Haiti where no infrastructure had previously been in place. Outcomes reported reflected a standardized approach to the management of NCDs in pregnancy. We provided valuable insights on areas to prioritize for the development and implementation of care delivery models for NCDs in pregnancy in LMIC. Our study had some limitations. Only 59% of women delivered in hospital and those who delivered outside of our health care facility may have experienced different outcomes. Moreover, neonatal glucose levels were not measured in all infants and the true prevalence of neonatal hypoglycemia was likely underestimated. We could not measure the impact of our intervention on maternal and neonatal outcomes since data prior to the DYAMAN study initiation was not available. We may have been underpowered to detect differences between groups for rare outcomes, such as neonatal hypoglycaemia. Our study population was a sample of pregnant women attending the antenatal clinic of a regional hospital in Saint-Marc and results may not be generalizable to the rest of the country’s pregnant population.
In conclusion, we showed that NCDs in pregnancy in Haiti were prevalent, challenging to care for, and important drivers of adverse maternal and perinatal outcomes. Further research is needed to assess local barriers, including economic barriers, to follow-up and treatment adherence during antepartum management of NCDs. Moreover, barriers to treatment escalation for maternal hyperglycemia, including physicians’ and patients’ experiences and perspectives must be explored. Identifying methods to increase health care contact after 36 weeks of gestation may partially prevent maternal and perinatal adverse outcomes resulting from late presentation to care. Finally, for gestational DM care delivery programs to be sustainable, the development of local capacity to supply diagnostic testing must be prioritized. The WHO established NCDs as a priority in the Global Health Action Plan through their “Strategy for Prevention and Control of NCD’s 2013-2020” [34]. Despite a global recognition of this pandemic, NCDs in low-income countries being the recipients of only 1.3% of development assistance for health in 2015 remain grossly underfunded [35]. We call for greater funding of research initiatives and health care delivery programs aimed at combatting health inequities disproportionately affecting women and children globally.














