Respiratory syncytial virus (RSV) is the leading cause of viral pneumonia and bronchiolitis, especially in younger children. The global estimate of RSV cases in children in 2015 was 33.1 million, with 3.2 million of these requiring hospitalisation [1]. The global estimated age-specific death rates due to RSV pneumonia in 2010 was 3.5 per 100 000 population, with RSV contributing 6.7% and 1.6% of all deaths in the 28-364 day-old and in the 1-4 year-old age groups, respectively [2]. Although most RSV studies to date have focused on children, the burden of RSV infection in adults, particularly in the older age group, is increasingly recognised [3-6]. Both asymptomatic and mild infection in adults raises the possibility that they may act as a source of paediatric infections that could be reduced through future vaccination [7].
Recently, RSV has been receiving more attention as one of the major viral respiratory pathogens causing acute lower respiratory infections in younger children. RSV epidemiological data, including incidence and prevalence, were estimated from different sources, including scientific publications and hospital reports. These data are usually limited to a specific location, community or hospital, during a specific time period, and therefore it is often difficult to conclude prevalence figures or estimate their accuracy. Several systematic reviews have tried to determine the burden of RSV infection, especially in children, in different regions in the world [1,8-10]. RSV epidemiological data are required to properly plan resource allocation and public health policies for disease control, particularly when or if a vaccine becomes available. In the future, surveillance that incorporates laboratory diagnostic testing using molecular techniques should provide information about transmission, evolution and the emergence of new RSV genotypes and strains that will help in establishing preventive measures to control RSV infection. The World Health Organization (WHO) has started piloting RSV surveillance in 14 countries since mid-2017 with the objective to obtain evidence-based data which will help in developing RSV vaccination policy [11].
The WHO Western Pacific Region (WPR), which consists of 37 countries in Asia, Oceania and the Pacific, is very diverse regarding demographics, political and socio-economic status, and includes health care systems that vary in terms of funding and resilience. The population of the WHO WPR area is approximately 1.9 billion people, which is approximately 25% of the global population [12]. There are an estimated 0.11 pneumonia episodes per child-year, with 61 900 pneumonia-related deaths annually in the WPR area [13]. The contribution of viral pathogens in acute lower respiratory tract infections in the region has, to date, mostly been provided by influenza data, as surveillance for Influenza is already conducted in 15 WPR countries [14]. However, the burden of RSV infections in the WPR region, in both adults and children is still unclear.
We reviewed RSV studies conducted in the WPR countries from January 2000 to October 2017 to investigate the epidemiological aspects of RSV: (incidence), prevalence, seasonality and hospitalisation status, and the associated molecular epidemiology data. To the best of our knowledge, this is the first review to systematically analyse RSV-related epidemiological data in the WPR countries.
Search strategy
Searches were systematically carried out in the main international literature databases (MEDLINE, EMBASE,Web of Science and SCOPUS), using the search terms: “RSV” OR “respiratory syncytial virus” AND “prevalence” OR “positivity” OR “rate” OR “infection” OR “proportion” OR “frequency” in combination with each WPR country (n = 37) using the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [15]. The date of the search was 30th October 2017. The final reference list of the articles included was reviewed for additional information. We also searched the web pages of all countries’ Ministries of Health and the WHO and WPR Office websites.
Selection criteria
Studies were included if the following criteria were fulfilled: 1) studies in humans; 2) studies in patients with acute respiratory tract infection (ARTI) or lower respiratory tract infection (LRTI) or influenza-like illness (ILI); 3) studies that reported one or more of the following: incidence, prevalence, seasonality, or molecular epidemiology (genotypes); and 4) studies published in English. Studies published or reported between January 2000 and October 2017 were included. We excluded articles if they were: 1) basic science or animal studies, 2) replication of studies, 3) nosocomial cases of RSV, or 4) case reports.
Data extraction and analysis
Literature screening was performed by assessing title, keyword and abstract. EndNote X7.5 (Thomson Reuters) was used for bibliography management, in which duplicates were removed before the initial screening. The first screening after removing 1027 duplicates was performed by excluding the irrelevant abstracts. Full-text screening was then conducted on 370 articles and a total of 196 papers were eligible for the study. The following data from eligible studies were extracted: study location (city, country); years and duration; setting (inpatient, outpatient); participant age; number of individuals and/or respiratory samples tested; number of RSV-positive specimens; number of each type of RSV-positive specimens (RSV A and RSV B); and number of genotypes identified.
We conducted meta-analyses using the Der-Simonian and Laird method using random-effects models [16] to calculate the pool of the RSV proportions by country, inpatient/outpatient, age, and RSV detection methods. The random-effects model was used because we assumed that the true estimates vary from study to study. All analyses were performed using MedCalc software version 19.05 (MedCalc Software, Belgium).
Primary and secondary outcomes
This systematic review included the following outcomes: (i) incidence and prevalence of RSV infections, (ii) percentage of ILIs and LRTIs being caused by RSV by demographic (age, country) and hospitalisation status (inpatients vs outpatients), and (iii) seasonality and molecular epidemiology.
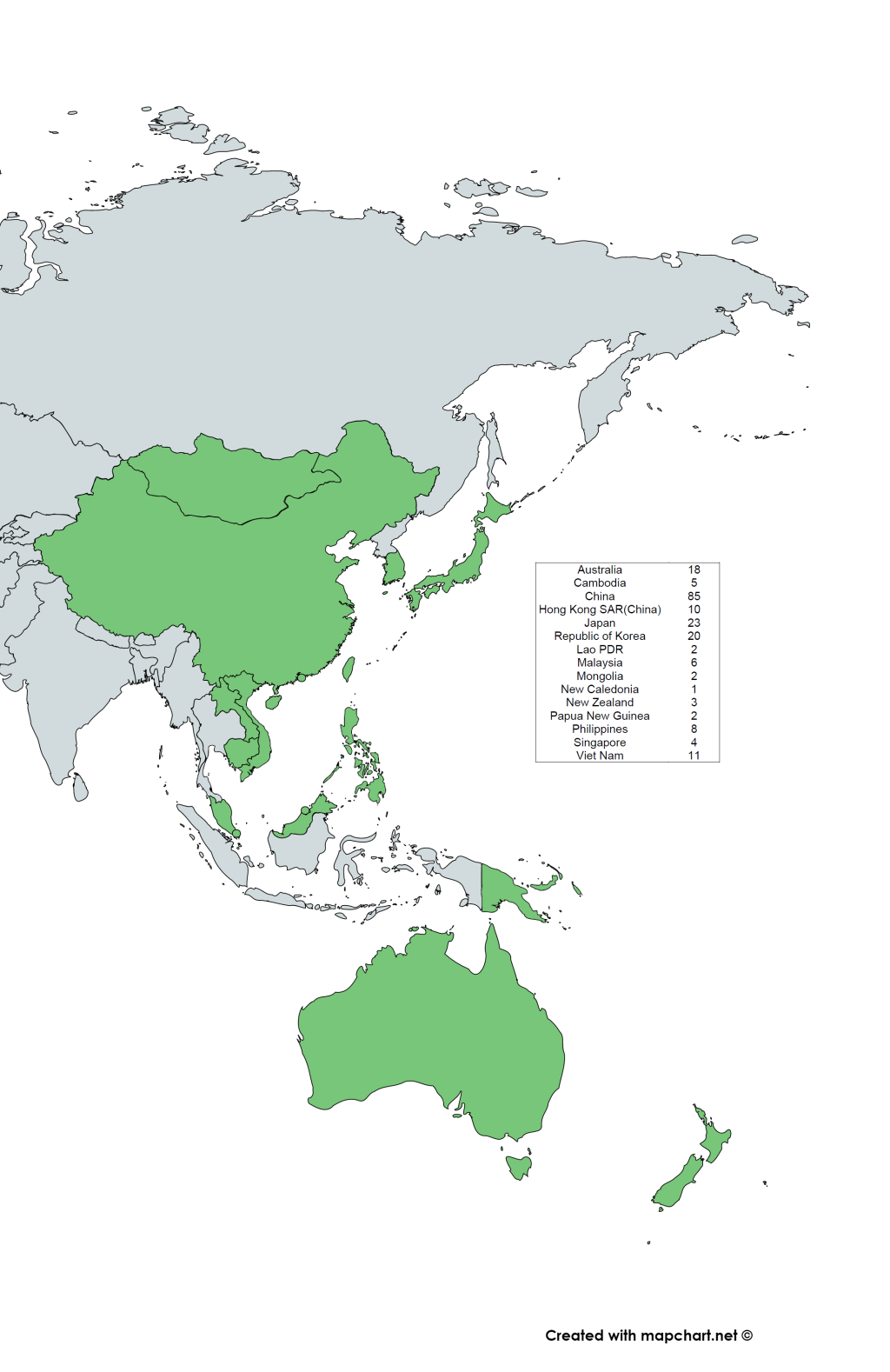
We identified 2072 articles, from which 196 studies were included based on our eligibility criteria (Figure 1), with two studies covering three WPR countries [18,19]. These included studies were only from 15 of the 37 countries listed in the WHO WPR. The map of WHO WPR countries and the distribution of the included RSV studies are shown in Figure 2. Of the 196 studies, 193 studies reported RSV-positivity rate, 13 studies reported the incidence, 95 studies reported seasonality and 43 studies reported RSV sub-grouping. The detailed information about the articles included in this study is provided in Table S1 in the Online Supplementary Document.
RSV positive rate
A total of 193 studies reported on the percentage of respiratory samples taken from patients with ARTI, LRTI or ILI that were positive for RSV, giving an overall positivity rate of 16.73% (95% confidence interval (CI) = 15.12%-18.4%) (Table 1). From the 196 studies that were included, 140 studies were conducted in children, 18 studies in adults, 38 studies covered all ages. The classification of age group (children and adult) was based on the classification used by each article. Of these studies, we then divided the age population into two categories (adult and children) by further extracting the data from the all-ages population to allow comparison of the positivity rate between the two categories. Among 868 236 children, RSV was detected in 120 137 (20.7%, 95% CI = 18.68%-22.83%); and among 54 915 adults, RSV was detected in 1003 (1.87%, 95% CI = 1.37%-2.46%). The RSV positivity rate in children is significantly higher than in adults (χ2 = 6535, P < 0.001, Table 1). Furthermore, the RSV positivity rate in children aged less than 5 years is higher than children older than 5 years (25.51%, 95% CI = 22.92%-28.19% vs 5.24%, 95% CI = 3.36%-7.51%, P < 0.001).
| No. articles included | Total No. patients | No. RSV positive | % RSV positive (95% CI) | ||
|---|---|---|---|---|---|
| Country | |||||
| Australia | 16 | 244 370 | 33 860 | 14.21 (8.06-21.76) | |
| Cambodia | 5 | 11 152 | 1002 | 8.80 (4.13-14.9) | |
| China (excl. HK) | 85 | 508 283 | 70 495 | 15.69(13.7-17.78) | |
| Hong Kong SAR (China) | 10 | 143 942 | 10 193 | 9.55 (3.4-18.35) | |
| Japan | 23 | 37 690 | 6533 | 24.74 (15.81-34.9) | |
| Malaysia | 6 | 76 525 | 3686 | 15.76 (4.45-32.24) | |
| Viet Nam | 11 | 12 273 | 2135 | 15.97 (8.68-24.96) | |
| Philippines | 8 | 33 242 | 3336 | 20.23 (8.17-35.97)) | |
| New Zealand | 2 | 1601 | 681 | 50.13 (29.19-71.04) | |
| Republic of Korea | 20 | 80 633 | 6389 | 18.05 (13.84-22.67) | |
| Mongolia | 2 | 542 | 52 | 13.03 (2.36-30.4) | |
| Singapore | 4 | 52 156 | 9196 | 13.58 (9.64-18.06) | |
| Lao PDR | 2 | 675 | 199 | 26.72 (5.88-55.6) | |
| Papua New Guinea | 2 | 380 | 44 | 14.74 (3.99 - 30.65) | |
| New Caledonia* | 1 | 108 | 49 | 45.37(35.76 - 55.24) | |
| Total | 197† | 1 203 572 | 147 850 | 16.73 (15.12-18.4) | |
| Age group: | |||||
| Children | 159† | 868 236 | 120 137 | 20.7(18.68-22.8) | |
| <5 y | 48 | 184 609 | 42 206 | 25.51 (22.92-28.19) | |
| >5 y | 16 | 11 393 | 501 | 5.24 (3.36-7.51) | |
| Adult | 34 | 54 915 | 1003 | 1.87 (1.37 - 2.46) | |
| Hospitalisation: | |||||
| Inpatient | 155‡ | 968 885 | 118 937 | 18.28(16.3-20.3) | |
| Outpatient | 43‡ | 159 574 | 17 702 | 11.54(8.97-14.37) | |
| Virus detection methods§: | |||||
| PCR | 121† | 307 184 | 40 897 | 16.04 (14.04 -18.15) | |
| IF | 29 | 459 315 | 42 229 | 17.7 (13.93-21.9) | |
| Other | 17 | 93 985 | 21 532 | 22.5 (17.35-28.14) | |
| Culture Mix | 29 | 334 925 | 42 381 | 15.65 (11.42–20.41) | |
RSV – respiratory syncytial virus, CI – confidence interval, y – year
*CI was not calculated.
†Two studies covered three countries.
‡One study covered three countries.
§Other-serology/Enzyme Immunoassay (EIA), Culture Mix- combination of culture and any methods, PCR – polymerase chain reaction, IF – immunofluorescence.
From the 193 studies that reported the prevalence of RSV based on the hospitalisation setting (inpatient, outpatient, or both), we classified the data into two categories, inpatients and outpatients, for analysis. There were 146 studies reporting RSV in hospitalised patients, 34 reporting RSV in outpatients, and 13 reporting RSV in both outpatients and inpatients. We also further extracted the data from these 13 articles to obtain each outpatients and inpatients data. RSV prevalence was higher in hospitalised inpatients (18.28%, 95% CI = 16.29%-20.4%) than in outpatients (11.89%, 95% CI = 9.22%-14.74%) (χ2 = 180, P < 0.001, Table 1). In this study, RSV prevalence of hospitalised children is higher than in outpatient cases (22.39%, 95% CI = 19.8%-25.1% vs 19.9%, 95% CI = 15.44%-24.77%, P < 0.001), while in outpatient adults, the positivity rate is higher than in hospitalised adults (3.11%, 95% CI = 1.36%-5.54% vs 1.43%, 95% CI = 0.79%-2.26%, P < 0.62).
RSV incidence
There were 15 studies reported the RSV incidence in eight countries/areas (Australia, China, Hong Kong SAR, Mongolia, New Zealand, Philippines, Singapore, Viet Nam), mostly in hospitalised children, while the outpatient incidence from ILI patients was reported in five countries (Table 2). The incidence of RSV-associated hospitalisation ranged between 4.9-30.9 per 1000 child-years and varied according to age group. There was only one study that provided the incidence of RSV-associated hospitalisation in adult patients, which was 0.57 per 1000 person-years [26]. The estimated incidence of RSV-associated ILI in children ranged from 0 to 137 per 1000 child-years [19,32].
| Country | Study period | Study Design | Age | Case definition | Sample size | RSV-associated hospitalization | RSV-associated ILI | RSV Incidence | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Australia | 1997-2004 | Retrospective case records | Children | LRTI, RSV +ve | 271 | 22/1000 live birth | _ | _ | [20] |
| 2000-2004 | Retrospective case records | <2 y | LRTI, RSV +ve | 173 | 20.4 per 1000 children <2y | _ | _ | [21] | |
| 1991-2000 | Published national laboratory data | <5 y | LRTI, RSV +ve | NA | _ | _ | 110.0-226.5 per 1000 children under 5 to 435.0-869.0 per 1000 infants | [22] | |
| 2001-2010 | Retrospective cohort | <5 y | LRTI | 16,119 | 4.9 per 1000 CY (<5 y), 25.6 per 1000 child-years (<3 mo) | _ | _ | [23] | |
| 2010-2011 | Prospective cohort | 6 m-10 y | ILI | 82 | _ | 0 per 1000 PY (6-11 mo), 2.1 per 1000 PY (12-23 mo), 1.1 per 1000 PY (24-35 mo), 0.95 per 1000 PY (36-59 mo), 0.25 per 1000 PY (>60 mo) | _ | [19] | |
| 2000-2012 | Retrospective cohort | <5 y | LRTI | 43,627 | _ | 2.5 per 1000 PY | _ | [24] | |
| China | 2011 | Cross-sectional | <5 y | SARI | 511 | 45 per 1000 children | _ | _ | [25] |
| Hong Kong | 1998-2012 | Cross-sectional | All ages | ARI | 19,405 | 15.8 per 1000 PY (<5 y), 0.57 per 1000 PY (>65 y) | _ | _ | [26] |
| 2003-2006 | Prospective cohort | <6 m | ARI | 1,031 | 23.3-31.1 per 1000 children | _ | _ | [27] | |
| Mongolia | 2013-2015 | prospective cohort study | Adult and infants under 6 mo | ILI and SARI | 1260 (adult), 1340 (<6 mo) | _ | pregnant women: 0.3 per 1000 PY | _ | [28] |
| New Zealand | 1995-2006 | Cross-sectional | All ages | ARI | NA | _ | _ | Monthly annualized incidence rate was 48.4 per 100 000 pop year | [29] |
| Philippines | 2010-2011 | Prospective cohort study | 6 m - 10 y | ILI | 724 | _ | 8.1 per 1000 PY (6-11 mo), 3.1 per 1000 PY (12-23 mo), 1.5 per 1000 PY (24-35 mo) 0.6 per 1000 PY (36-59 mo), 0.07 per 1000 PY (>60 mo) | _ | [19] |
| 2012-2014 | Cross-sectional | All ages | ILI and SARI | ILI: 6267, SARI:2962 | 1.9 per 1000 person | 1.4 per 1000 person | _ | [30] | |
| Singapore | 2010-2011 | Prospective cohort study | 6 m - 10 y | ILI | 34 | _ | 0 per 1000 PY (12-23 mo), 2.5 per 1000 PY (24-35 mo) 0.5 per 1000 PY (36-59 mo), 0.1 per 1000 PY (>60 mo) | _ | [19] |
| Viet Nam | 2007-2010 | Cross-sectional | 1 mth-5 y | ARI | 1786 | 23.4 per 1000 children <2y, 9.59 per 1000 children <5y | _ | _ | [31] |
| 2009-2010 | Prospective cohort study | less than 2 y | ARI | 2549 | 8 to 30.9 per 1000 IYO | 23.2 to 137 per 1000 IYO | _ | [32] | |
| 2010-2012 | Cross-sectional | 0-5 y | ARI | 1854 | 5.89 to 15.47 per 1000 children <5 PY | _ | _ | [33] |
ARI – acute respiratory infection, CY – child-years, LRTI – lower respiratory tract infection, ILI- influenza like illness, PY – person-years, SARI – severe acute respiratory infection, IYO – infant-years of observation, mo – months, y – years
Seasonality
One hundred and nineteen of the 196 studies reported the data related with the seasonality of RSV, including the duration, the week/month of the RSV season, and the peak of RSV activity (Table 3). Generally, temperate countries, both in the Northern and Southern hemispheres, experienced their peak of the epidemic in the winter. In subtropical and tropical countries, the cases peaked mostly in the rainy (wet) season. Several studies from countries that have a wide latitude range, such as China and Australia, have reported different times for the peaks of RSV epidemics depending on the latitude.
| No articles included | Seasonality | Peak or common months | |
|---|---|---|---|
| Southern hemisphere: | |||
| Australia | |||
| Temperate (NSW, Victoria) | 4 | Seasonal | Winter |
| Subtropical (Queensland) | 1 | Seasonal | Winter |
| Tropical (Queensland) | 1 | Seasonal | Rainy season |
| Tropical (Northern Territory) | 1 | Throughout the year | Rainy season |
| Desert (Northern Territory) | 2 | Throughout the year | Winter |
| New Zealand | 1 | Seasonal | Winter |
| Northern hemisphere | |||
| China | |||
| Central | 2 | Seasonal | Winter and Spring |
| Eastern | 7 | Seasonal | Winter |
| 7 | Seasonal | Winter and Spring | |
| 1 | Seasonal | Autumn and Winter | |
| 2 | Throughout the year | ||
| Northeastern | 4 | Seasonal | Winter |
| 1 | Seasonal | Winter and Spring | |
| Northwest | 5 | Seasonal | Winter and Spring |
| Southern | 2 | Throughout the year | Winter and Spring |
| 4 | Throughout the year | Two peaks (winter to spring, summer to autumn) | |
| 2 | Seasonal | Winter | |
| 1 | Seasonal | Spring and summer | |
| 2 | Seasonal | Spring | |
| Western | 2 | Seasonal | Winter |
| Japan | 5 | Seasonal | Winter and Autumn |
| 3 | Seasonal | Winter | |
| South Korea | 8 | Seasonal | Winter |
| 3 | Seasonal | Winter and Autumn | |
| 2 | Seasonal | Winter and Spring | |
| Mongolia | 1 | Seasonal | Winter |
| Hong Kong | 3 | Throughout the year | Rainy season |
| 3 | Two peaks | Winter-Spring, Summer-Autumn | |
| Tropical | |||
| Cambodia | 2 | Throughout the year | Rainy season |
| Viet Nam | 5 | Throughout the year | Rainy season |
| 3 | Seasonal | Hot and dry season | |
| Laos | 2 | Throughout the year | Rainy season |
| Malaysia | 4 | Throughout the year | Rainy season |
| New Caledonia | 1 | Seasonal | Transition from wet to dry season |
| Philippines | 3 | Throughout the year | Rainy season |
| Singapore | 2 | Throughout the year | Rainy season |
RSV – respiratory syncytial virus, WPR – Western Pacific Region
RSV genotypes
Forty-three studies reported the RSV subgrouping (RSV A and B). From the total 13 775 RSV cases that underwent subgrouping, RSV A was identified in 8829 cases (63.43%, 95% CI = 57.34%-69.31%) and RSV B in 4272 cases (30.87%, 95% CI = 25.74%-36.25%), respectively. There were 51 RSV cases from seven studies with concurrent RSV A and B infection.
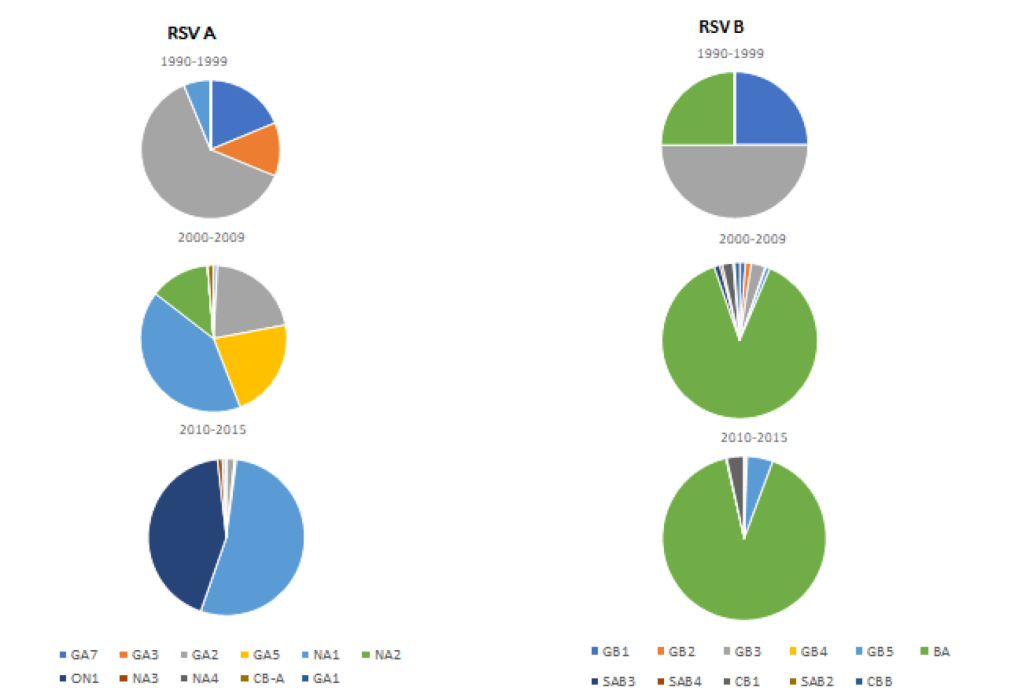
The distribution of RSV genotypes between 1990-2015 was reported in 34 studies from eight WPR countries. Of these 34 studies, we used 33 studies that performed genotyping based on the G protein gene. Eleven RSV A and RSV B genotypes were identified from 3957 RSV cases (2841 RSV A and 1116 RSV B). The distribution of each genotype is shown in Figure 3. NA1 and BA genotypes were the predominant genotypes reported for RSV A and B, respectively. Japan, China and Malaysia had the most complete data as they had performed multi-year molecular epidemiology studies (Table S2 in the Online Supplementary Document).
Recently, RSV has received more attention as one of the major viral respiratory pathogens that cause significant mortality and morbidity, especially in younger children [2]. Several groups have determined the global burden of RSV infection in children by conducting a series of systematic reviews. The first systematic review and meta-analysis of global RSV data covering 1995-2009 estimated that, in 2005, the incidence of RSV-associated ARTI in children aged less than 5 years was 22%, resulting in 3%-9% of all deaths, most of which (90%) occurred in low- to middle-income countries [8]. Further analysis, using more recent publications and data, estimated that 28% of ARTI episodes were RSV-associated, causing 13%-22% of all ARTI mortality in children aged under 5 years in 2015 [1]. A meta-analysis of pneumonia-related data conducted by the Global Action Plan for Pneumonia and Diarrhoea (GAPPD) group found that RSV is the most common aetiology of pneumonia in children, estimating that it contributes 29% of all episodes [34]. However, a systematic review of the adult population has rarely been performed.
In this review, the RSV pooled positivity rate is 16.73% of all acute respiratory infection cases in the WPR countries. The previous estimate based just on pneumonia episodes showed a higher RSV prevalence than our result [34]. Our result shows that the RSV-positive rate in both adult and child patients is higher than a previous systematic review of studies in Africa with a pooled prevalence of RSV infection in ARTI cases of 14.6% [10]. However, our RSV pooled prevalence is lower than the prevalence from systematic review studies in Latin America and Iran, where the RSV pooled prevalence was 18.7% and 9.2%-41.5%, respectively [9,35]. The RSV positivity rate within the countries in the WPR ranged from 8.8% (Cambodia) to 50.13% (New Zealand). Our results show that the RSV prevalence in both adult and child patients in China of 15.6% (95% CI = 13.7%-17.78%) is similar to a previous systematic review where it was 18.7% (95% CI = 17.1%-20.5%) [17].
The difference in RSV prevalence may be due to differences in the number of studies conducted within different countries, with most of the studies identified being from China, Japan, the Republic of Korea and Australia. These four countries have established strong national influenza surveillance programmes, which can be easily extended to cover other respiratory viruses [36]. Many of the studies in the WPR region were from the expansion of influenza-like illness (ILI) and severe acute respiratory infection (SARI) surveillance. Japan commenced RSV surveillance in 2003 [37], while the Korean Influenza and Respiratory Virus Surveillance System (KINRESS) for major respiratory viruses commenced in 2005, with sentinel sites located nationwide [38]. China has a nationwide influenza-surveillance system, but RSV surveillance is less well-established [36,39]. In Australia there is no national RSV surveillance system, however, there is monitoring of the trends of detected infections through several laboratories [22].
The RSV surveillance pilot project conducted by the WHO is based on the Global Influenza Surveillance and Response System and aims to provide evidence-based data, including on the epidemiology and circulation of RSV, that is needed for the implementation and monitoring of any future RSV vaccine [40]. This surveillance uses the existing influenza surveillance platforms that incorporate laboratory identification and molecular characterisation to monitor circulating genotypes and identify emerging viruses. This recent effort supports the Asia Pacific Strategy for Emerging Diseases (APSED), developed by WHO WPR and South East Asian Region (SEAR) member countries, that aims to guide member countries in achieving their obligations under the International Health Regulations (IHR 2005) [41]. Furthermore, the RSV surveillance pilot project may facilitate research on respiratory viruses as proposed by the BRaVE (Battle against Respiratory Viruses) initiative in 2013 [42].
The largest population group included in our RSV study is children with acute respiratory tract infections, 159 studies, which show a prevalence higher than the adult group. Our results are similar to previous systematic studies in both developing countries [9,10] and developed countries [43,44]. The prevalence of RSV in adults is still not well-established compared with studies of adult influenza infection and the limited number of studies in adults might contribute to the lower reported prevalence in this age population. Based on our study, RSV has a considerable contribution to the WPR as an aetiology of respiratory infection, especially in children less than 5 years. Our analysis of the difference in the RSV positivity rate between children under 5 years and greater than 5 years is concordant with the results from African region [10]. As the specific prevention measure of an RSV vaccine is not yet available, other respiratory infection prevention and control measures targeted at this age group should be implemented in both developed and developing countries.
Most studies included in this review were conducted in hospital settings, with the percentage of RSV detected in inpatients being higher than in outpatients. Our results are similar to other studies where RSV prevalence was higher in hospitalised patients than in outpatients with ILI [43,45]. The RSV positivity rate in previous studies in hospitalised children varied between 3%-29% [46], which were similar to our result (22.38%). However, our study found that the RSV positivity rate in outpatient adults was higher than in hospitalised adults, which is consistent with a study conducted in the United States demonstrating a higher rate of RSV emergency department visits than RSV hospitalisation rates in adults [47].
The reported incidence of acute respiratory infection in children due to RSV in the WPR countries is broad. The incidence of RSV-associated hospitalisation in children decreased with increasing age, a finding similar to previous studies [48-50]. A systematic review of children hospitalised due to RSV found that the incidence in the western countries (Australia, USA and Europe) was higher than in Asia [49], while in our study the incidence between western countries and Asia is similar. A study from Egypt reported a higher average annual incidence of RSV in outpatients than in hospitalised inpatients [51], while in the WPR countries only one study from Viet Nam showed similar results [32]. The variability of incidence has also been discussed in other studies possibly due to location, case definition, study population and diagnostic methods [49]. Our study provides additional data regarding the RSV burden in the outpatient setting to that in western countries [50]. As there are few studies of the acute respiratory RSV infection incidence in adults in the WPR, future research should focus on this age group.
The seasonality of infectious diseases, especially viral respiratory infections, is important for planning public health responses and workforce distribution, as seen for global influenza surveillance. Our results showed similar RSV seasonality that covered several locations that were not included in the previous review [52]. In the temperate regions in both the Northern and Southern hemispheres, RSV peaked in the winter season. In tropical countries, the seasonality of RSV is not clearly defined. However, the number of cases seems to peak at different times of the year, mostly in the rainy season. In Australia, the seasonality of RSV differs by region with the northern tropical part, including Cairns and Darwin, experiencing an RSV peak in the rainy season such as seen in neighbouring tropical countries [53].
Diagnostic methods for detecting respiratory viruses were advanced recently by the development of molecular methods for determining infectious aetiology. In this analysis, 121 studies used RSV polymerase chain reaction (PCR) assays to identify the respiratory viruses present during the acute respiratory infection. The positivity rate in the studies using PCR was similar to the studies using immunofluorescence (IF), which might be related to the specificity and sensitivity of the IF techniques [10]. A previous systematic review that included only studies using PCR had a higher RSV prevalence than studies using IF and immunochromatographic methods [9,10,35. As we observed, there were reports of co-infection or co-detection of RSV with other respiratory viruses in the specimens tested, which were assumed to be due to the increased sensitivity of molecular methods [54-56].
Molecular methods have recently replaced monoclonal antibody determination of the grouping of RSV into RSV A and RSV B [57]. Recent studies analysed the distribution of RSV groups and genotypes and demonstrated that both RSV A and RSV B co-circulate in the same epidemic period with shifting predominance of the groups [58-61]. Further molecular characterisation studies identified various genotypes within each group, with each genotype able to cluster temporally, locally or circulate at different times and different global locations [58]. The molecular epidemiology studies conducted in the WHO WPR countries demonstrated the co-circulation of RSV A and RSV B with shifting dominance occurring between groups and changes to the diversity of the genotypes. RSV A was more dominant than RSV B in the WPR countries during the past two decades. This finding is concordant with studies conducted in other parts of the world [59-62].
Several studies in the WPR countries included sequencing to differentiate specific genotypes and examine their circulation, estimate pathways of transmission, and detect new and emerging genotypes. Japan, China, and Malaysia had relatively comprehensive molecular data over more extended periods. However, these molecular studies from large countries are often limited to a few sentinel sites and therefore may not be representative of the whole country’s disease burden. RSV genotype circulation in the WPR countries followed a similar pattern to global circulation [63], as can be observed by the emergence of the new genotypes, BA (RSV B) and ON1 (RSV A). The RSV B BA genotype, first detected in specimens from Buenos Aires in 1999 [64], was first identified in the WPR from Malaysia’s specimens in 1999 [65]. The BA genotype then further identified in several countries in the WPR region and became the dominant RSV B genotype. The distribution of RSV A ON1 genotype in the WPR countries also followed a similar pattern as in other global regions. The ON1 genotype was first identified in specimens from Ontario in 2010 [66] and emerged in 2011 in the WPR countries; in China [67], Malaysia [65] and Republic of Korea [68]. In the Southern hemisphere, the molecular epidemiology of RSV is not well-established as studies from Australia and New Zealand are limited. As an RSV vaccine is currently under development, data on the distribution of RSV strains will be important both for the composition of the vaccine and for post-vaccine implementation, particularly with regard to any immune selection pressure exerted by the vaccine.
Our study has some limitations. First, there were variations in the case definitions, study design, and pathogen detection methods used in these included studies. We had difficulty in classifying the children’s age groups, as each countries’ studies had differing age ranges. Therefore, the interpretation of the results has been cautious due to the difficulties in comparing some of these studies. In the future, it would be desirable to define the age-ranges of the children and adults when conducting epidemiology studies globally. Second, RSV is not the main focus of several studies, as most studies focused on finding other possible causes of respiratory infections. Third, the advances in PCR technology with increased sensitivity, which incorporate the detection of multiple respiratory viruses, allows for the inclusion of RSV, where it may not be the active infection. Therefore, as in the previous systematic review (10), we should consider the presence of viral co-infections in our future results. Lastly, the amount of data available per country is variable, with only half of the WPR countries having reported RSV and multiple studies from certain countries, which may bias the data, Despite this, our study has attempted to summarise data on the epidemiology of RSV in both adults and children in the WPR.
This study suggests that the RSV has considerable prevalence in the WPR countries, although the RSV data are limited as several countries have focused more on infections in children and hospitalised patients. The seasonality and the molecular epidemiology of RSV among WPR countries are similar to and reflect the global pattern. Further studies and surveillance incorporating molecular laboratory typing in adults and out-patients are needed to determine the overall burden of RSV infection in the WPR countries.














