The burden of sexually transmitted infections (STIs) and sequelae remains a major global health concern [1]. Nearly one million persons are infected with a curable STI every day [2], and about half a billion are living with herpes simplex virus type 2 (HSV-2) [3]. The largely asymptomatic nature of STIs, particularly for women, leaves most individuals unaware of their infection [1]. STIs have been associated with HIV acquisition [4-6], and poor reproductive health outcomes including pelvic inflammatory disease, ectopic pregnancy, infertility, and perinatal deaths [1,7].
Commercial heterosexual sex networks (CHSNs) are believed to play a critical role in STI transmission [8-10]. STIs have been demonstrated as proxy biomarkers of sexual risk behaviour [11,12], and as a powerful tool for understanding the structure of sexual networks and predicting HIV epidemic potential [11-13]. However, unlike HIV, STI epidemiology in CHSNs remains, globally, a neglected area of research [1]. Programmatically, STI surveillance among female sex workers (FSWs) continues to be weak and infection levels poorly quantified [1]. Sexual propagation of STIs along CHSNs is also poorly understood given the dearth or limited validity of self-reported sexual behaviour data [13-15].
To attend to the United Nations’ Sustainable Development Goals (SDGs) and targets [16], particularly SDG3 target of “ensuring universal access to sexual and reproductive health services” [16], and to reduce the global burden of disease attributed to STIs, the World Health Organization (WHO) has recently formulated the “Global Health Sector Strategy on STIs” [6]. The goal of this strategy is to eliminate STIs as a major public health concern by 2030 through an integrated approach for prevention and control [6]. Milestones for 2020 include achieving 70% coverage for comprehensive STI prevention services among key populations [6]. The strategy’s first strategic direction entails “understanding the STI epidemic as a basis for advocacy, political commitment, national planning, resource mobilization and allocation, implementation, and programme improvement” [6].
Despite remarkable progress in HIV research [17], and an understanding of the role of FSWs [18], people who inject drugs (PWID) [19], and men who have sex with men (MSM) [20], in the HIV epidemic in the Middle East and North Africa (MENA) region, the epidemiology of STIs and the role of CHSNs in driving STI transmission remain largely unknown [21]. The two global reviews of STI epidemiology in FSWs had no data for any of the 23 MENA countries [22,23]. A large volume of STI data in the region resides in databases that were never analyzed, or in country-level reports that were never published in the scientific literature [24,25].
Against this background, our study aimed to characterize the epidemiology of key STIs among FSWs in MENA by 1) systematically reviewing and synthesizing all available published and unpublished evidence for Treponema pallidum (henceforth referred to as syphilis), Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, and HSV-2 incidence and/or prevalence, 2) estimating, for each STI, the pooled mean prevalence of current and/or ever (seropositivity using antibody testing) infection, and 3) identifying sources of between-study heterogeneity, and regional and temporal trends associated with STI prevalence.
We conducted a systematic review and an in-depth quantitative assessment to characterize STI epidemiology among FSWs in MENA. Details of the study methodology (including specific statistical analyses) can be found in subsequent sections.
Search strategy and selection criteria
Evidence for syphilis, C. trachomatis, N. gonorrhaoeae, T. vaginalis, and HSV-2 immunoglobulin G (IgG) incidence and/or prevalence among FSWs in MENA was systematically reviewed, informed by Cochrane’s Collaboration guidelines [26]. Findings were reported following Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [27] (checklist in Table S1 in Online Supplementary Document). The MENA definition covers 23 countries—Afghanistan, Algeria, Bahrain, Djibouti, Egypt, Iran, Iraq, Jordan, Kuwait, Lebanon, Libya, Morocco, Oman, Pakistan, Palestine, Qatar, Saudi Arabia, Somalia, Sudan, Syria, Tunisia, United Arab Emirates (UAE), and Yemen—based on convention in HIV research [19,20,24,25], and definitions of WHO, Joint United Nations Programme on HIV/AIDS (UNAIDS), and World Bank [24].
Systematic searches were performed up to September 20, 2018, on international databases (PubMed and Embase), regional and national databases (WHO Global Health Observatory data repository [28], WHO African Index Medicus database, WHO Index Medicus for the Eastern Mediterranean Region database, Iranian Scientific Information Database, Iraqi Academic Scientific Journals’ database, and Pakistan’s PakMediNet database), abstract archives of International AIDS Society Conferences [29], as well as published and unpublished country-level and international organizations’ reports available through the MENA HIV/AIDS Epidemiology Synthesis Project database [24,25]. Search strings were broad (MeSH/Emtree terms exploded to cover all subheadings and free text terms) with no language or year restrictions (Box S1 in Online Supplementary Document).
Duplicate citations were identified using a reference manager, Endnote. Titles and abstracts were then screened for relevance, with relevant/potentially relevant citations undergoing full-text screening. Any document reporting an incidence and/or prevalence measure in FSWs for an STI of interest, based on primary data, was eligible for inclusion. Case reports, case series, editorials, commentaries, and reviews were excluded. Hand searching was further performed on reference lists of all relevant articles.
The term ‘study’ is used here to refer to a specific STI incidence or prevalence measure in a specific FSW population. Accordingly, one document/report could contribute multiple studies and one study could be published in different reports. Duplicate study results were included only once using the more detailed/recent report.
Data extraction and synthesis
Extraction was performed by HC, and double extraction by AS (extraction list in Box S2 in Online Supplementary Document). Discrepancies were settled by consensus, or by contacting authors. Full-texts in languages other than English were extracted by native speakers. Data were stratified by infection type (current vs ever (seropositivity using antibody testing)), and summarized using medians, ranges, and interquartile ranges (IQR). Definitions of infection types and details of the classification of diagnostic methods’ results into current, recent, and ever infection can be found in Table S2 in Online Supplementary Document. It was assumed, for N. gonorrhoeae and T. vaginalis studies, whenever a diagnostic method was not explicitly specified, that the diagnostic method assessed current infection.
All STI studies were extracted and reported. However, studies applying the same assay to different biological specimens from the same person were included only once in analyses, for statistical independence. This was done based on a sequential order that prioritized infection detection in endocervical swabs, followed by vaginal, then urine samples. Studies assessing prevalence using different diagnostic methods, were also included only once in analyses, with studies using polymerase chain reaction prioritized over those using culture or other methods.
Quality assessment
The quality assessment for each STI prevalence study was informed by Cochrane Collaboration guidelines (criteria in Table S3 in Online Supplementary Document) [30]. Studies were classified as having “low” vs “high” risk of bias (ROB) on each of three quality domains assessing the 1) rigor of sampling methodology (probability-based; non-probability-based), 2) response rate (≥60% or ≥60% of target sample size reached for studies using respondent-driven or time-location sampling; <60%), and 3) STI ascertainment (biological assay explicitly indicated; otherwise). Studies with missing information for a specific domain were classified as having “unclear” ROB for that domain.
Given reported limitations in HSV-2 diagnostics [31,32], the quality of HSV-2 assays was determined by consulting with an expert advisor, Professor Rhoda Ashley-Morrow, University of Washington, Seattle. Studies where the validity of the diagnostic method could not be confirmed, were excluded from the systematic review.
Quality domains were included in meta-regression analyses (described below) to assess their impact on prevalence.
Meta-analyses
For each STI, the pooled mean prevalence of current and/or ever infection, along with the corresponding 95% confidence intervals (CIs), were estimated using meta-analysis. Overall prevalence measures were replaced by their strata where applicable. For each study, one final stratification was considered based on a pre-defined sequential order that prioritizes country of origin, followed by type of FSW, year, region, and age. Subregional and time-trend analyses were conducted as warranted by data. Variances were stabilized using Freeman-Tukey type arcsine square-root transformation [33,34]. Weights were applied using the inverse-variance method [34,35], before pooling measures using a Dersimonian-Laird random-effects model [36], thereby accounting for sampling variation and for true heterogeneity [37]. Missing sample sizes for measures or their strata (<4% of all studies) were imputed using the median sample size, as calculated from studies with available information.
Heterogeneity assessment used Cochran’s Q statistic to confirm existence of heterogeneity across studies, I2 to determine magnitude of between-study variation that is due to true differences in effect size (prevalence) rather than chance, and prediction intervals to estimate the 95% interval of the true effect sizes’ distribution [37,38].
Meta-analyses were implemented in R 3.4.2 (R core team, Vienna, Austria) [39].
Meta-regressions
Only syphilis had a considerable number of measures (>100) to warrant conduct of random-effects meta-regression analyses. Independent variables considered a priori were: country/subregion, year of data collection, infection type, diagnostic method, STI ascertainment, sample size, sampling methodology, and response rate. Details of subgrouping and justifications are in Table S4 in Online Supplementary Document. Meta-regression was conducted using the log-transformed odds of syphilis infection and corresponding variance. Factors associated with higher odds of infection at P ≤ 0.10 in univariable analyses were included in the multivariable analysis. Factors with P ≤ 0.05 in the multivariable model were considered as significant predictors of heterogeneity in syphilis prevalence.
Meta-regressions were implemented in Stata/SE 15.1 (StataCorp, College Station, TX, USA) [40].
Search results and scope of evidence
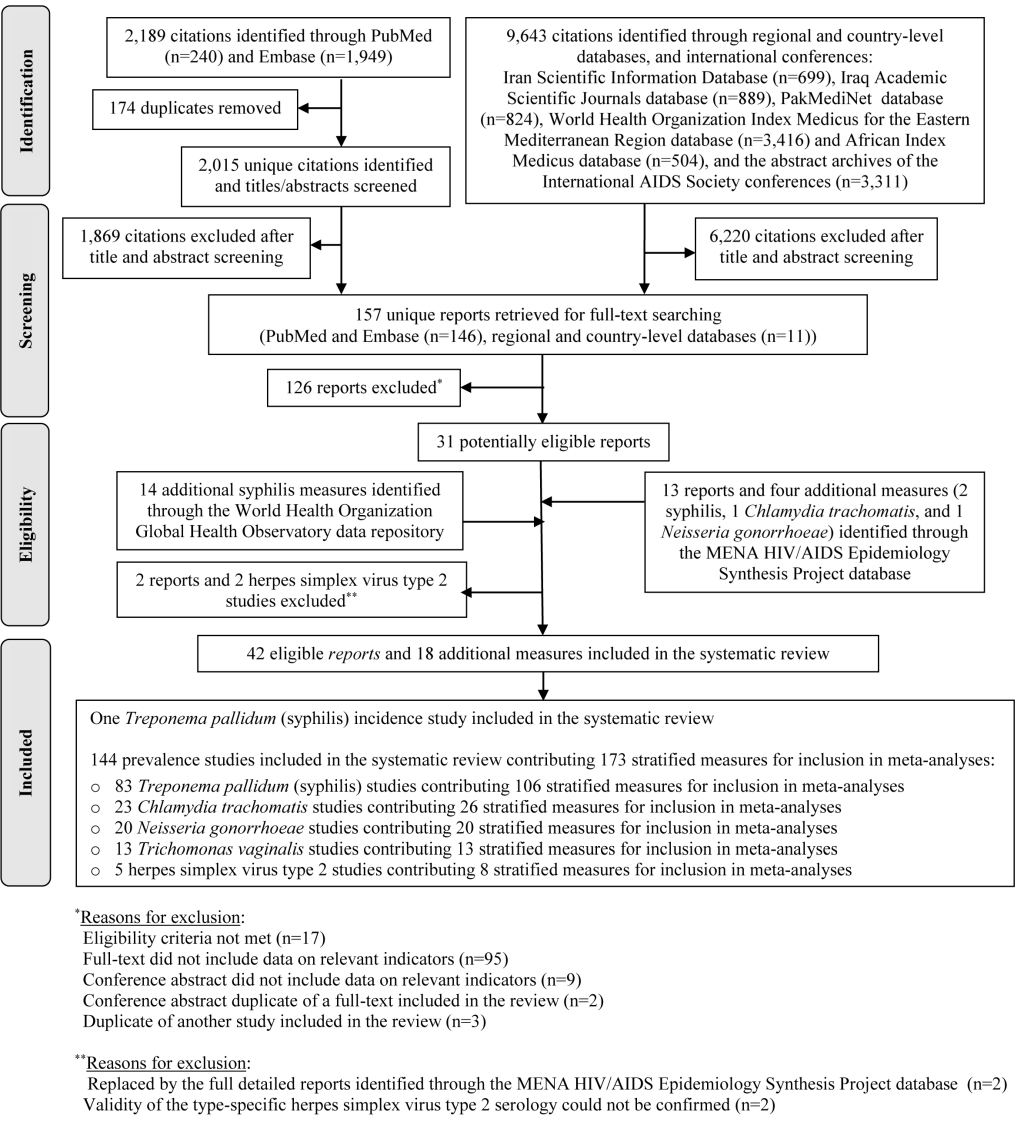
Figure 1 shows the study selection process based on PRISMA. The search identified a total of 11 832 citations: 240 through PubMed, 1949 through Embase, and 9643 through the rest of the databases. After removing duplicates and screening of titles and abstracts, 157 reports qualified for full-text screening, of which 31 were eligible for inclusion in the systematic review.
Thirteen additional reports, two of which replaced eligible articles, and four additional STI measures, were further identified through the MENA HIV/AIDS Epidemiology Synthesis Project database. Fourteen additional syphilis prevalence measures were identified through the WHO Global Health Observatory data repository. Two studies were excluded based on consultation with Professor Rhoda Ashley-Morrow, an expert advisor in HSV-2 diagnostics, because the validity of the type-specific HSV-2 serology could not be confirmed [41,42].
In sum, 42 eligible reports and 18 additional STI measures were included in the systematic review. These yielded one syphilis incidence study, and 144 prevalence studies assessing the different STIs. The latter contributed 173 stratified measures for inclusion in meta-analyses and meta-regressions.
STI prevalence data were available for 45 812 FSWs from 13 of the 23 MENA countries. Nearly two-thirds (58.9%) of prevalence studies assessed syphilis (in 29 769 FSWs), 16.3% assessed C. trachomatis (in 5613 FSWs), 12.8% assessed N. gonorrhoeae (in 5230 FSWs), 8.5% assessed T. vaginalis (in 4258 FSWs), and 3.6% assessed HSV-2 IgG (in 942 FSWs). Most studies (80.8%) were conducted post-2000. Over half (51.1%) of studies reported on current infection, 30.5% on ever infection (seropositivity using antibody testing), and 1.4% on recent infection. Time of exposure was unclear for the rest of studies (17.0%).
Incidence studies
The only one identified incidence study assessed syphilis incidence in FSWs. The study was conducted in 1988 in Mogadishu, Somalia, and reported cumulative incidence at 12.5% after six months of follow-up [43].
Prevalence studies
Prevalence of current syphilis infection among FSWs ranged, across studies (n = 28), from 0%-50.8%, with a median of 9.4% (IQR = 3.0%-23.4%; Table 1). Meanwhile, seropositivity for syphilis (n = 33) antibodies ranged from 0%-69.0%, with a median of 4.2% (IQR = 1.9%-15.2%).
| Country short citation | Year(s) of data collection | City/province | Sampling | Study site | Assay type | Tested (n) | Prevalence (%) |
|---|---|---|---|---|---|---|---|
| CURRENT INFECTION | |||||||
| Afghanistan: | |||||||
| Todd, 2010 [44] | 2006-08 | Jalalabad, Kabul, Mazar-i-Sharif | Conv | NGO | RPR+ & TPHA+ | 520 | 0 |
| Egypt: | |||||||
| MOH, 2000 [45] | 1999-00 | Greater Cairo | Conv | Community | RPR+ & TPHA+ | 52 | 5.8 |
| Iran: | |||||||
| Kassaian, 2012 [46] | 2009-10 | Isfahan | Conv | Prison, drop-in center | RPR+ | 91 | 0 |
| Navadeh, 2012 [42] | 2010 | Kerman | RDS | Community | VDRL+ | 139 | 7.2 |
| Kazerooni, 2014 [41] | 2010-11 | Shiraz | RDS | Community | VDRL+ & FTA-ABS+ | 278 | 0 |
| Jahanbakhsh, 2017 [47] | 2012 | Tehran | Conv | Homeless shelters | RPR+ | 14 | 0 |
| Morocco: | |||||||
| MOH, 2008 [48] | 2007 | Agadir, Rabat-Sale, Tanger | Conv | Clinic | VDRL+ & TPHA+ | 141 | 13.5 |
| MOH, 2012 [49] | 2011-12 | Agadir | RDS | Community | VDRL+ & TPHA+ | 362 | 21.4 |
| MOH, 2012 [49] | 2011-12 | Fes | RDS | Community | VDRL+ & TPHA+ | 359 | 18.8 |
| MOH, 2012 [49] | 2011-12 | Rabat | RDS | Community | VDRL+ & TPHA+ | 392 | 13.9 |
| MOH, 2012 [49] | 2011-12 | Tanger | RDS | Community | VDRL+ & TPHA+ | 318 | 13.3 |
| Pakistan: | |||||||
| Baqi, 1998 [50] | 1993-94 | Karachi | Conv | Red-light district | VDRL+ & FTA-ABS+ | 81† | 5.0 |
| Rehan, 2009 [51] & NACP, 2005 [52] | 2004 | Karachi | Snowball | Community | RPR+ & TPHA+ | 421 | 3.6 |
| Rehan, 2009 [51] & NACP, 2005 [52] | 2004 | Lahore | SyCS | Red-light district | RPR+ & TPHA+ | 387 | 16.0 |
| Shah, 2004 [53] | 2004 | Hyderabad | Conv | Community | VDRL+ & TPHA+ | 157 | 11.5 |
| Hawkes, 2009 [54] | 2007 | Abbottabad | RDS | Community | RPR+ & TPHA+ | 107 | 2.8 |
| Hawkes, 2009 [54] | 2007 | Rawalpindi | RDS | Community | RPR+ & TPHA+ | 426 | 1.2 |
| Khan, 2011 [55] | 2007 | Lahore | RDS | Community | RPR+ & TPHA+ | 730 | 4.5 |
| Somalia: | |||||||
| Jama, 1987 [56] | 1985-86 | Mogadishu | Conv | Community | VDRL+ & TPHA+ | 85 | 44.7 |
| Jama Ahmed, 1991 [43] | 1988-89 | Mogadishu | Conv | Community | VDRL/RPR+ & TPHA+ | 155 | 47.7 |
| Scott, 1991 [57] | 1989 | Kismayu, Merca | Conv | NR | RPR+ & FTA-ABS+ | 57 | 50.8 |
| Corwin, 1991 [58] | 1990 | Chismayu, Merca, Mogadishu | Conv | NR | RPR+ & FTA-ABS+ | 302 | 35.4 |
| Watts, 1994 [59] | 1990 | Chismayu, Merca, Mogadishu | Conv | NR | RPR+ & FTA-ABS+ | 236 | 30.9 |
| IOM, 2017 [60] | 2014 | Hargeisa | RDS | Community | RDT+ & RPR+ | 96 | 2.4 |
| Sudan: | |||||||
| MOH, 2016 [61] | 2015-16 | Juba, South Sudan | RDS | Community | RDT+ & RPR+ | 832 | 7.3 |
| Tunisia: | |||||||
| Bchir, 1988 [62] | 1987 | Sousse | Conv | NR | VDRL+ & TPHA+ | 42 | 28.6 |
| Ayachi, 1997 [63] | 1992-94 | Tunis | Conv | NR | VDRL+ & TPHA+ | 79 | 24.1 |
| Yemen: | |||||||
| Stulhofer, 2008 [64] | 2008 | Aden | RDS | Community | VDRL+ | 244 | 4.9 |
| EVER INFECTION‡ | |||||||
| Afghanistan: | |||||||
| NACP, 2010 [65] | 2009 | Kabul | RDS | Community | RDT+ | 368 | 5.4 |
| NACP, 2012 [66] | 2012 | Herat | RDS | Community | RDT+ | 344 | 0.9 |
| NACP, 2012 [66] | 2012 | Kabul | RDS | Community | RDT+ | 333 | 0.0 |
| NACP, 2012 [66] | 2012 | Mazar-i-Sharif | RDS | Community | RDT+ | 355 | 2.0 |
| Algeria: | |||||||
| MOH, 2009 [67] | 2004 | National | Conv | Sentinel surveillance | TPHA+ | 185 | 11.9 |
| MOH, 2009 [67] | 2007 | National | Conv | Sentinel surveillance | TPHA+ | 380 | 18.4 |
| Iran: | |||||||
| Mirzazadeh, 2016 [68] | 2015 | National | Conv | Community, clinic | RDT+ | 1,337 | 0.4 |
| Pakistan: | |||||||
| Hawkes, 2009 [54] | 2007 | Abbottabad | RDS | Community | TPHA+ | 107 | 2.8 |
| Hawkes, 2009 [54] | 2007 | Rawalpindi | RDS | Community | TPHA+ | 426 | 1.6 |
| Bibi, 2010 [69] | 2003 | Hyderabad | Conv | Red-light district | TPHA+ | 50 | 44.0 |
| Raza, 2015 [70] | 2014 | Rawalpindi | Conv | Clinic | RDT+ | NR | 20.0 |
| Somalia: | |||||||
| Jama, 1987 [56] | 1985-86 | Mogadishu | Conv | Community | TPHA+ | 85 | 57.6 |
| Jama Ahmed, 1991 [43] | 1988-89 | Mogadishu | Conv | Community | TPHA+ | 155 | 69.0 |
| Burans, 1990 [71] | NR | Mogadishu | Conv | NR | TPHA+ | 89 | 28.1 |
| IOM, 2017 [60] | 2008 | Hargeisa | RDS | Community | RDT+ | 237 | 3.4 |
| Sudan: | |||||||
| Sudan NACP, 2012 [72] | 2011 | Alshamalia | RDS | Community | RDT+ | 305 | 1.5 |
| Sudan NACP, 2012 [72] | 2011 | Blue Nile | RDS | Community | RDT+ | 279 | 3.4 |
| Sudan NACP, 2012 [72] | 2011 | Gadarif | RDS | Community | RDT+ | 282 | 3.4 |
| Sudan NACP, 2012 [72] | 2011 | Gezira | RDS | Community | RDT+ | 296 | 5.4 |
| Sudan NACP, 2012 [72] | 2011 | Kassala | RDS | Community | RDT+ | 288 | 4.3 |
| Sudan NACP, 2012 [72] | 2011 | Khartoum | RDS | Community | RDT+ | 287 | 1.7 |
| Sudan NACP, 2012 [72] | 2011 | North Darfur | RDS | Community | RDT+ | 303 | 5.2 |
| Sudan NACP, 2012 [72] | 2011 | North Kodofan | RDS | Community | RDT+ | 296 | 4.1 |
| Sudan NACP, 2012 [72] | 2011 | Red Sea | RDS | Community | RDT+ | 293 | 8.9 |
| Sudan NACP, 2012 [72] | 2011 | River Nile | RDS | Community | RDT+ | 291 | 1.9 |
| Sudan NACP, 2012 [72] | 2011 | Sinnar | RDS | Community | RDT+ | 303 | 5.3 |
| Sudan NACP, 2012 [72] | 2011 | South Darfur | RDS | Community | RDT+ | 299 | 1.8 |
| Sudan NACP, 2012 [72] | 2011 | West Darfur | RDS | Community | RDT+ | 284 | 1.8 |
| Sudan NACP, 2012 [72] | 2011 | White Nile | RDS | Community | RDT+ | 288 | 4.2 |
| MOH, 2016 [61] | 2015-16 | Juba, South Sudan | RDS | Community | RDT+ | 832 | 12.0 |
| Tunisia | |||||||
| Bchir, 1988 [62] | 1987 | Sousse | Conv | NR | TPHA+ | 42 | 38.1 |
| Ayachi, 1997 [63] | 1992-94 | Tunis | Conv | NR | TPHA+ | 79 | 36.7 |
| Znazen, 2010 [73] | 2007 | Gabes, Sousse, Tunis | Conv | Clinic | TPHA+ | 183 | 2.7 |
| UNCLEAR | |||||||
| Afghanistan: | |||||||
| WHO, 2018 [28] | 2010 | NR | NR | NR | NR | NR | 8.7 |
| MENA HIV ESP, 2013 [74] | 2012 | Kabul | NR | NR | NR | 440 | 5.7 |
| WHO, 2018 [28] | 2017 | NR | NR | NR | NR | 2,457 | 1.3 |
| Algeria: | |||||||
| WHO, 2018 [28] | 2013 | Oran | NR | NR | NR | 27 | 7.4 |
| WHO, 2018 [28] | 2014 | Saida | NR | NR | NR | 24 | 29.2 |
| WHO, 2018 [28] | 2016 | NR | Conv | VCT | NR | 183 | 14.2 |
| WHO, 2018 [28] | 2017 | NR | Conv | VCT | NR | 81 | 16.0 |
| Djibouti: | |||||||
| WHO, 2015 [1] | 2014 | 4 urban sites | NR | NR | NR | 361 | 5.0 |
| Iran: | |||||||
| WHO, 2018 [28] | 2008 | NR | NR | NR | NR | NR | 1.6 |
| Moayedi-Nia, 2016 [75] | 2012-13 | Tehran | RDS | Community | NR | 161 | 0 |
| Jordan: | |||||||
| WHO, 2015 [1] | 2008 | NR | NR | NR | NR | NR | 6.7 |
| Morocco: | |||||||
| Khattabi, 2005 [76] | 2004 | National | Conv | Prison | NR | 332 | 9.6 |
| Khattabi, 2005 [76] | 2004 | National | Conv | Clinic | NR | 272 | 12.1 |
| Khattabi, 2005 [76] | 2004 | Grand Casablanca | Conv | STI clinic | NR | 143 | 9.0 |
| Bennani, 2006 [77] | 2005 | National | Conv | Prison | NR | 102 | 11.8 |
| Bennani, 2006 [77] | 2005 | National | Conv | Clinic | NR | 143 | 13.3 |
| WHO, 2018 [28] | 2008 | NR | NR | NR | NR | NR | 16.9 |
| Pakistan: | |||||||
| MENA HIV ESP, 2010 [24] | 2007 | NR | NR | NR | NR | NR | 23.5 |
| Somalia: | |||||||
| WHO, 2018 [28] | 2017 | Bossaso, Hargeisa, Mogadishu | RDS | Community | NR | 860 | 2.7 |
| Sudan: | |||||||
| WHO, 2018 [28] | 2016 | National | RDS | Community | NR | 4,123 | 4.1 |
| WHO, 2018 [28] | 2017 | South Sudan | NR | NR | NR | 1,244 | 14.4 |
| Yemen: | |||||||
| WHO, 2018 [28] | 2010 | Hodeida | RDS | Community | NR | 301 | 0 |
Conv – convenience, FTA-ABS – fluoresceent treponemal antibody absorption test, IOM – International Organization for Migration, MENA HIV ESP – MENA HIV/AIDS Epidemiology Synthesis Project database, MOH – Ministry of Health, NACP – National AIDS Control Program, NGO – non-governmental organization, NR – not reported, RDS – respondent-driven sampling, RDT – rapid diagnostic test, RPR – rapid plasma regain, STI – sexually transmitted infection, SyCS – systematic cluster sampling, TPHA – Treponema pallidum haemagglutination assay, VCT – voluntary counseling and testing center, VDRL – venereal disease research laboratory
*The table is sorted, for each country, by data collection year(s) then city/province.
†Sample comprised of 77 FSWs and 4 transgender women.
‡Ever infection indicates seropositivity using antibody testing.
Current C. trachomatis infection prevalence (n = 14) ranged from 0.7%-72.9%, with a median of 7.7% (IQR = 1.7%-22.4%), while seropositivity prevalence using IgG (n = 5) ranged from 19.8%-100%, with a median of 85.8% (IQR = 46.8%-97.1%; Table 2). Two studies reported recent C. trachomatis infection (assessed using serological biomarkers) at 29.2% [79] and 95.0% [78].
| Country short citation | Year(s) of data collection | City/province | Sampling | Study site | Specimen | Assay type | Tested (n) | Prevalence (%) |
|---|---|---|---|---|---|---|---|---|
| CURRENT INFECTION | ||||||||
| Chlamydia trachomatis | ||||||||
| Algeria: | ||||||||
| Kadi, 1989 [78] | NR | NR | Conv | Clinic | Endocervical | IFAT | 44 | 45.5 |
| Egypt: | ||||||||
| MOH, 2000 [45] | 1999-00 | Cairo | Conv | Community | Urine | NAAT | 52 | 7.7 |
| Iran: | ||||||||
| Darougar, 1983 [79] | NR | Bandar Abbas, Tehran | Conv | Clinic | Endocervical | Culture | 116 | 6.9 |
| Kazerooni, 2014 [41] | 2010-11 | Shiraz | RDS | Community | Vaginal | NAAT | 278 | 9.0 |
| Mirzazadeh, 2016 [68] | 2015 | National | Conv | Clinic, community | Vaginal | NAAT | 1337 | 6.0 |
| Morocco: | ||||||||
| MOH, 2008 [48] | 2007 | Agadir, Rabat Sale, Tanger | Conv | Clinic | Endocervical & urine | NAAT | 141 | 22.7 |
| MOH, 2012 [49] | 2011-12 | Agadir | RDS | Community | Endocervical | NAAT | 368 | 22.4 |
| Pakistan: | ||||||||
| Rehan, 2009 [51] | 2004 | Karachi | Snowball | Community | Vaginal | NAAT | 348 | 5.2 |
| Rehan, 2009 [51] | 2004 | Lahore | SyCS | Red-light district | Vaginal | NAAT | 283 | 11.0 |
| Hawkes, 2009 [54] | 2007 | Abbottabad | RDS | Community | Endocervical | NAAT | 107 | 0.9 |
| Hawkes, 2009 [54] | 2007 | Rawalpindi | RDS | Community | Endocervical | NAAT | 426 | 1.7 |
| Khan, 2011 [55] | 2007 | Lahore | RDS | Community | Endocervical | NAAT | 730 | 7.7 |
| Somalia: | ||||||||
| IOM, 2017 [60] | 2014 | Hargeisa | RDS | Community | Urine | NAAT | 90 | 0.7 |
| Tunisia: | ||||||||
| Znazen, 2010 [73] | 2007 | Gabes, Sousse, Tunis | Conv | Clinic | Endocervical | NAAT | 188 | 72.9 |
| Neisseria gonorrhoeae | ||||||||
| Egypt: | ||||||||
| MOH, 2000 [45] | 1999-00 | Cairo | Conv | Community | Urine | NAAT | 52 | 7.7 |
| Iran: | ||||||||
| Kazerooni, 2014 [41] | 2010-11 | Shiraz | RDS | Community | Vaginal | Culture | 278 | 1.4 |
| Navadeh, 2012 [42] & WHO, 2011 [80] | 2010 | Kerman | RDS | Community | NR | NR† | 144 | 0 |
| Nasirian, 2017 [81] | 2013-14 | Isfahan | Conv | Harm reduction | Endocervical | NAAT | 99 | 9.1 |
| Nasirian, 2017 [81] | 2013-14 | Isfahan | Conv | Harm reduction | Urine | NAAT | 99 | 0‡ |
| Taghizadeh, 2015 [82] | 2014 | Sari | Conv | Drop-in center | NR | NR† | 117 | 1.0 |
| Mirzazadeh, 2016 [68] | 2015 | National | Conv | Clinic, community | Vaginal | NAAT | 1337 | 1.3 |
| Morocco: | ||||||||
| MOH, 2008 [48] | 2007 | Agadir, Rabat Sale, Tanger | Conv | Clinic | Endocervical & urine | NAAT | 141 | 10.6 |
| MENA HIV ESP, 2010 [24] | NR | NR | NR | NR | NR | NR† | NR | 3.5 |
| MOH, 2012 [49] | 2011-12 | Agadir | RDS | Community | Endocervical | NAAT | 368 | 11.7 |
| Pakistan: | ||||||||
| Rehan, 2009 [51] | 2004 | Karachi | Snowball | Community | Vaginal | NAAT | 348 | 9.8 |
| Rehan, 2009 [51] | 2004 | Lahore | SyCS | Red-light district | Vaginal | NAAT | 383 | 12.3 |
| Hawkes, 2009 [54] | 2007 | Abbottabad | RDS | Community | Endocervical | NAAT | 107 | 1.9 |
| Hawkes, 2009 [54] | 2007 | Rawalpindi | RDS | Community | Endocervical | NAAT | 426 | 2.0 |
| Khan, 2011 [55] | 2007 | Lahore | RDS | Community | Endocervical | NAAT | 730 | 7.5 |
| Somalia: | ||||||||
| Burans, 1990 [71] | NR | Mogadishu | Conv | NR | NR | Culture | 89 | 11.2 |
| IOM, 2017 [60] | 2014 | Hargeisa | RDS | Community | Urine | NAAT | 91 | 0.4 |
| Tunisia: | ||||||||
| NACP, 2005 [83] | 2005 | NR | NR | NR | NR | NR† | NR | 12.0-17.0§ |
| Znazen, 2010 [73] | 2007 | Gabes, Sousse, Tunis | Conv | Clinic | Endocervical | Culture | 188 | 3.7‖ |
| Znazen, 2010 [73] | 2007 | Gabes, Sousse, Tunis | Conv | Clinic | Endocervical | NAAT | 188 | 11.2 |
| Trichomonas vaginalis | ||||||||
| Egypt: | ||||||||
| MOH, 2000 [45] | 1999-00 | Cairo | Conv | Community | Urine | NAAT | 52 | 19.2 |
| Iran: | ||||||||
| Vafaei, 2015 [84] | 2009-11 | Shiraz | Conv | Clinic, drop-in center | Endocervical | Wet mount | 85 | 8.2 |
| Navadeh, 2012 [42] & WHO, 2011 [80] | 2010 | Kerman | RDS | Community | NR | NR† | 144 | 1.4 |
| Nasirian, 2017 [81] | 2013-14 | Isfahan | Conv | Harm reduction | Endocervical | NAAT | 99 | 0.0 |
| Nasirian, 2017 [81] | 2013-14 | Isfahan | Conv | Harm reduction | Urine | NAAT | 99 | 0.0‡ |
| Mirzazadeh, 2016 [68] | 2015 | National | Conv | Clinic, community | Vaginal | NAAT | 1337 | 11.9 |
| Morocco: | ||||||||
| MOH, 2008 [48] | 2007 | Agadir, Rabat Sale, Tanger | Conv | Clinic | Endocervical & vaginal | Culture | 141 | 14.9 |
| MOH, 2012 [49] | 2011-12 | Agadir | RDS | Community | Vaginal | NAAT | 367 | 11.8 |
| Pakistan: | ||||||||
| Rehan, 2009 [51] | 2004 | Karachi | Snowball | Community | Vaginal | Culture | 386 | 5.2 |
| Rehan, 2009 [51] | 2004 | Lahore | SyCS | Red-light district | Vaginal | Culture | 384 | 19.3 |
| Hawkes, 2009 [54] | 2007 | Abbottabad | RDS | Community | Vaginal | NAAT | 107 | 5.7 |
| Hawkes, 2009 [54] | 2007 | Rawalpindi | RDS | Community | Vaginal | NAAT | 426 | 4.3 |
| Khan, 2011 [55] | 2007 | Lahore | RDS | Community | Vaginal | Culture | 730 | 5.1 |
| RECENT INFECTION | ||||||||
| Chlamydia trachomatis | ||||||||
| Algeria: | ||||||||
| Kadi, 1989 [78] | NR | NR | Conv | Clinic | Serum | MIF>1:64¶ | 44 | 95.0 |
| Iran: | ||||||||
| Darougar, 1983 [79] | NR | Bandar Abbas, Tehran | Conv | Clinic | Serum | MIF-IgM | 154 | 29.2 |
| EVER INFECTION** | ||||||||
| Chlamydia trachomatis | ||||||||
| Algeria: | ||||||||
| Kadi, 1989 [78] | NR | NR | Conv | Clinic | Serum | MIF-IgG | 44 | 100 |
| Iran: | ||||||||
| Darougar, 1983 [79] | NR | Bandar Abbas, Tehran | Conv | Clinic | Serum | MIF-IgG | 154 | 94.2 |
| Kassaian, 2012 [46] | 2009-10 | Isfahan | Conv | Drop-in center | Serum | ELISA-IgG | 91 | 19.8 |
| Tunisia | ||||||||
| Bchir, 1988 [62] | 1987 | Sousse | Conv | NR | Serum | MIF>1:16 | 42 | 73.8 |
| Znazen, 2010 [73] | 2007 | Gabes, Sousse, Tunis | Conv | Clinic | Serum | MIF-IgG | 183 | 85.8 |
| UNCLEAR: | ||||||||
| Chlamydia trachomatis | ||||||||
| Iran: | ||||||||
| Navadeh, 2012 [42] & WHO, 2011 [80] | 2010 | Kerman | RDS | Community | NR | NR | 144 | 2.9 |
| Morocco: | ||||||||
| MENA HIV ESP, 2010 [24] | NR | NR | NR | NR | NR | NR | NR | 19.1 |
Conv – convenience, ELISA – enzyme-linked immunosorbent assay, IFAT – indirect immunofluorescence antibody test, IgG – immuneglobulin G, IgM - immunoglobulin M, IOM – International Organization for Migration, MENA HIV ESP – MENA HIV/AIDS Epidemiology Synthesis Project database, MIF – micro-immunofluorescence, MOH – Ministry of Health, NAAT – Nucleic acid amplification test, NR – not reported, RDS – respondent-driven sampling, SyCS – systematic cluster sampling, WHO – World Health Organization
*The table is sorted for each country by data collection year(s) then city/province.
†For Neisseria gonorrhoeae and Trichomonas vaginalis studies, whenever the diagnostic method was not explicitly specified, it was assumed that the diagnostic method assessed current infection.
‡Studies reported in the systematic review, but not included in analyses considering the priority order followed for selecting studies applying the same assay to different biological specimens.
§Range reported based on several studies whose abstracts or full-texts could not be retrieved (mid-point: 14.5%).
‖Studies reported in the systematic review, but not included in analyses as prevalence was also assessed using NAAT.
¶Reported in study as recent infection.
**Ever infection indicates seropositivity using antibody testing.
Current N. gonorrhoeae infection prevalence (n = 18) ranged from 0%-14.5%, with a median of 7.6% (IQR = 1.3%-11.1%; Table 2). Current T. vaginalis infection prevalence (n = 12) ranged from 0%-19.3%, with a median of 7.0% (IQR = 4.5%-14.2%; Table 2). HSV-2 seropositivity (using IgG; n = 5) ranged from 4.7%-55.5%, with a median of 20.0% (IQR = 6.4%-39.1%; Table 3).
| Country short citation | Year(s) of data collection | City/province | Sampling | Study site | Specimen | Assay type | Tested (n) | Prevalence (%) |
|---|---|---|---|---|---|---|---|---|
| Pakistan: | ||||||||
| Hawkes, 2009 [54] | 2007 | Abbottabad | RDS | Community | Serum | ELISA-IgG | 107 | 4.7 |
| Hawkes, 2009 [54] | 2007 | Rawalpindi | RDS | Community | Serum | ELISA-IgG | 426 | 8.0 |
| Syria: | ||||||||
| Ibrahim, 2000 [85] | 1995-98 | Damascus | Conv | Cheap hotels & prison | Serum | MEIA-IgG | 101 | 22.8 |
| Ibrahim, 2000 [85] | 1995-98 | Damascus | Conv | Bars | Serum | MEIA-IgG | 125 | 20.0 |
| Tunisia: | ||||||||
| Znazen, 2010 [73] | 2007 | Gabes, Sousse, Tunis | Conv | Clinic | Serum | ELISA-IgG | 183 | 55.5 |
Conv – convenience, ELISA – enzyme-linked immunosorbent assay. MEIA – micro-enzyme immunoassay. RDS – respondent-driven sampling
Quality assessment
The summarized and study-specific ROB assessments of prevalence measures are in Tables S5 and S6 in Online Supplementary Document, respectively. Briefly, nearly half of studies (44.7%) used probability-based sampling. Most studies (78.7%) indicated explicitly the biological assay used for STI ascertainment. Response rate information was missing in over half of studies (51.8%).
Overall, studies were of reasonable quality. Close to 60% of studies had low ROB on at least two quality domains, and none had high ROB on two or more domains.
Pooled mean prevalence estimates
Table 4 shows the results of meta-analyses estimating the pooled mean prevalence of current and/or ever infection for each STI. The mean prevalence of current infection was estimated at 12.7% (95% CI = 8.5%-17.7%) for syphilis, 14.4% (95% CI = 8.2%-22.0%) for C. trachomatis, 5.7% (95% CI = 3.5%-8.4%) for N. gonorrhoeae, and 7.1% (95% CI = 4.3%-10.5%) for T. vaginalis.
| Sexually transmitted infection* | Studies | Samples | Reported prevalence | Pooled mean prevalence | Heterogeneity measures | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N† | Tested | Positive | Median‡ (%) | Range‡ (%) | Estimate (%) | 95% CI | Q§ (P) | I2‖ (%; 95% CI) | Prediction interval¶ (95%) | |
| Current infection: | ||||||||||
| Treponema pallidum (syphilis) | 34 | 7103 | 842 | 10.8 | 0-62.0 | 12.7 | 8.5-17.7 | 1045.3 (P < 0.0001) | 96.8 (96.2-97.4) | 0.0-48.8 |
| Chlamydia trachomatis | 16 | 4608 | 512 | 8.4 | 0.7-76.2 | 14.4 | 8.2-22.0 | 611.4 (P < 0.0001) | 97.5 (96.9-98.1) | 0.0-53.6 |
| Neisseria gonorrhoeae | 20 | 5230 | 301 | 7.9 | 0-17.5 | 5.7 | 3.5-8.4 | 248.2 (P < 0.0001) | 92.3 (89.6-94.4) | 0.0-21.6 |
| Trichomonas vaginalis | 13 | 4258 | 397 | 7.1 | 0-19.3 | 7.1 | 4.3-10.5 | 164.7 (P < 0.0001) | 92.7 (89.3-95.0) | 0.0-23.7 |
| Recent infection: | ||||||||||
| Chlamydia trachomatis | 2** | 198 | 87 | 62.1 | 29.2-95.0 | – | – | – | – | – |
| Ever infection:†† | ||||||||||
| Treponema pallidum (syphilis) | 50 | 9968 | 710 | 7.0 | 0-92.3 | 12.8 | 9.4-16.6 | 1261.0 (P < 0.0001) | 96.1 (95.5-96.7) | 0.0-45.2 |
| Chlamydia trachomatis | 6 | 514 | 395 | 84.7 | 19.8-100 | 80.3 | 53.2-97.6 | 213.0 (P < 0.0001) | 97.7 (96.4-98.5) | 0.0-100.0 |
| Herpes simplex virus type 2 IgG | 8 | 942 | 188 | 20.3 | 4.7-59.7 | 23.7 | 10.2-40.4 | 185.0 (P < 0.0001) | 96.2 (94.3-97.5) | 0.0-84.9 |
| Unclear: | ||||||||||
| Treponema pallidum (syphilis) | 22 | 12 698 | 771 | 8.9 | 0-29.2 | 7.7 | 5.1-10.7 | 591.3 (P < 0.0001) | 96.4 (95.5-97.2) | 0.0-25.7 |
| Chlamydia trachomatis | 2** | 293 | 32 | 11.0 | 2.9-19.1 | – | – | – | – | – |
CI – confidence interval, FSWs – female sex workers, IgG – immunoglobulin G, P – P-value
*The same population may have contributed different measures for both current infection and ever (seropositivity using antibody testing) infection.
†Missing sample sizes for measures (or their strata) were imputed using the median sample size calculated from studies with available information (only two stratified measures for Neisseria gonorrhoeae, one stratified measure for Chlamydia trachomatis, one stratified measure for current syphilis infection, 5 stratified measures of unclear syphilis infection, had their sample size imputed, that is 5% of all data).
‡Medians and ranges were calculated based on the stratified prevalence measures.
§Q – the Cochran’s Q statistic is a measure assessing the existence of heterogeneity in effect size (here, prevalence) across studies.
‖I2 – a measure assessing the magnitude of between-study variation that is due to differences in effect size (here, prevalence) across studies rather than chance.
¶Prediction interval: a measure estimating the 95% interval of the distribution of true effect sizes (here, prevalence measures).
**Meta-analyses were performed if at least three studies were available.
††Ever infection indicates seropositivity using antibody testing.
The mean prevalence of ever infection was estimated at 12.8% (95% CI = 9.4%-16.6%) for syphilis, 80.3% (95% CI = 53.2%-97.6%) for C. trachomatis, and 23.7% (95% CI = 10.2%-40.4%) for HSV-2 IgG.
There was strong evidence for heterogeneity in effect size (here, prevalence). P for Cochran’s Q statistic was always <0.0001. I2 was >90% in all meta-analyses, indicating that most variability is due to true differences in effect size across studies, rather than being due to chance. Prediction intervals were also wide affirming high heterogeneity.
Additional meta-analyses at the subregional level indicated the mean prevalence of current syphilis infection at 3.0% (95% CI = 0.9%-9.2%) in Eastern MENA, 17.6% (95% CI = 14.2%-21.3%) in North Africa, and 27.8% (95% CI = 15.2%-42.4%) in the Horn of Africa (Table S7 in Online Supplementary Document). There was also a tendency for a decline in current infection prevalence post-2010 (Table S8 and Figure S1A in Online Supplementary Document). For the rest of the STIs, the number of studies was small and the CIs were wide and overlapping to warrant conclusive statement about the temporal trend (Table S8 in Online Supplementary Document).
Predictors of variability in syphilis infection
Country/subregion, year of data collection, diagnostic method, sample size, sampling methodology, and response rate were associated with higher odds of syphilis infection in the univariable meta-regression analyses. These were, therefore, included in the multivariable model (Table 5). About a third of the variability was explained by each of year of data collection and subregion (adjusted R-squared: 34.6% and 31.5%, respectively). Meanwhile, no evidence for an association with infection type (current infection; ever infection), or STI ascertainment (biological assay explicitly indicated; otherwise) was found.
| Factors | Studies | Samples | Univariable analyses | Multivariable analysis* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total N | Total n | OR† (95% CI) | P | P‡ of LR test | Variance explained R2 (%) | AOR† (95% CI) | P | P§ of LR test | ||
| Country/subregion:‖ | ||||||||||
| Eastern MENA | Afghanistan, Iran, Pakistan | 28 | 10 865 | 1.00 | <0.001 | 31.52 | 1.00 | <0.001 | ||
| Egypt, Jordan, Yemen | Egypt, Jordan, Yemen | 4 | 881 | 0.89 (0.15-5.10) | 0.893 | 0.66 (0.13-3.28) | 0.609 | |||
| North Africa | Algeria, Morocco, Sudan, Tunisia | 48 | 12 394 | 5.34 (2.45-11.61) | <0.001 | 5.01 (2.37-10.61) | <0.001 | |||
| Horn of Africa | Djibouti, Somalia, South Sudan | 26 | 5629 | 21.63 (8.89-52.69) | <0.001 | 6.40 (2.45-16.69) | <0.001 | |||
| Year of data collection¶ | 106 | 29 769 | 0.88 (0.85-0.91) | <0.001 | <0.001 | 34.61 | 0.93 (0.88-0.98) | 0.005 | 0.005 | |
| Infection type | Current | 34 | 7103 | 1.00 | 0.515 | 0.00 | – | – | – | |
| Ever** | 50 | 9968 | 1.25 (0.52-3.00) | 0.622 | – | – | – | |||
| Unclear | 22 | 12 698 | 0.69 (0.23-2.04) | 0.501 | – | – | – | |||
| Diagnostic method | RPR/VDRL & TPHA/FTA-ABS/RDT | 29 | 6095 | 1.00 | <0.001 | 22.44 | 1.00 | 0.444 | ||
| RPR/VDRL | 4 | 488 | 0.09 (0.01-0.61) | 0.013 | 0.76 (0.15-4.00) | 0.746 | ||||
| TPHA | 28 | 1781 | 2.17 (0.86-5.45) | 0.099 | 1.29 (0.54-3.07) | 0.558 | ||||
| RDT | 23 | 8707 | 0.17 (0.06-0.45) | <0.001 | 0.46 (0.18-1.18) | 0.104 | ||||
| Not specified | 22 | 12 698 | 0.43 (0.16-1.16) | 0.094 | 0.75 (0.24-2.33) | 0.614 | ||||
| STI ascertainment | Biological assay not reported | 23 | 13 066 | 1.00 | 0.284 | 0.15 | – | – | – | |
| Biological assay explicitly indicated | 83 | 16 703 | 1.66 (0.65-4.20) | 0.284 | – | – | – | |||
| Sample size | <100 participants | 42 | 1960 | 1.00 | <0.001 | 20.02†† | 1.00 | |||
| ≥100 participants | 64 | 27 809 | 0.16 (0.08-0.32) | <0.001 | 1.60 (0.62-4.15) | 0.329 | 0.329 | |||
| Sampling methodology | Non-probability/unclear sampling | 66 | 12 555 | 1.00 | <0.001 | 18.73‡‡ | 1.00 | |||
| Probability-based sampling | 40 | 17 214 | 0.16 (0.08-0.34) | <0.001 | 0.63 (0.25-1.63) | 0.339 | 0.339 | |||
| Response rate | <60%/unclear | 69 | 18 400 | 1.00 | <0.001 | 10.23§§ | 1.00 | |||
| ≥60% | 37 | 11 369 | 0.25 (0.12-0.54) | 0.001 | 0.73 (0.29-1.84) | 0.495 | 0.495 | |||
AOR – adjusted odds ratio, CI – confidence interval, FTA-ABS – fluorescent treponemal antibody absorption test, LR – likelihood ratio, OR – odds ratio, P – P-value, RDT – rapid diagnostic test, RPR – rapid plasma regain, STI – sexually transmitted infection TPHA – Treponema pallidum haemagglutination assay. VDRL – venereal disease research laboratory
*Adjusted R2 in the multivariable model: 48.46%.
†An increment of 0.1 was added to number of events when generating log odds of syphilis infection. This is because 8 stratified measures had zero events.
‡Factors with P ≤ 0.1 were eligible for inclusion in the multivariable analysis.
§Factors with P < 0.05 in the multivariable model were considered as significant predictors.
‖Countries were grouped based on geography and similarity in prevalence levels.
¶Missing values for year of data collection (only one stratified measure) were imputed using data for year of publication adjusted by the median difference between year of publication and median year of data collection for studies with complete information.
**Ever infection indicates seropositivity using antibody testing.
††The high R2 was investigated and found to be due to confounding with year of data collection. Most studies with sample size ≥100 were conducted in recent years.
‡‡The high R2 was investigated and found to be due to confounding with country and year of data collection. Studies with non-probability sampling were mostly from the Horn of Africa. These studies tended also to be conducted in earlier years.
§§The high R2 was investigated and found to be due to confounding with year of data collection. Most studies with response rate ≥60% were conducted in recent years.
The multivariable analysis showed strong evidence for subregional differences, with Horn of Africa and North Africa showing, respectively, 6-fold (adjusted odds ratio (AOR): 6.4; 95% CI = 2.5-16.7) and 5-fold (AOR = 5.0; 95% CI = 2.5-10.6), higher odds of syphilis infection than Eastern MENA.
There was also strong evidence for a temporal trend of decreasing odds of infection at 7% per year (AOR = 0.93; 95% CI = 0.88-0.98; linearity dictated by data (Figure S1 in Online Supplementary Document) over the last three decades. Although this trend was noted in all subregions, individual subregion meta-regressions were not always powered to detect statistical significance (not shown).
No evidence for an association with diagnostic method, sample size, sampling methodology, and response rate was identified in the multivariable model. The multivariable model explained 48.5% of variation in syphilis prevalence.
We provided, to our knowledge, the first detailed assessment of the epidemiology of key STIs in FSWs in MENA, a neglected key population. Our findings indicated substantial STI prevalence, several folds higher than that among the general population [2,13,24,86]. These findings suggest a major role for CHSNs in driving STI transmission in MENA. We further found large heterogeneity in syphilis infection levels by subregion within MENA, as well as a trend of decreasing odds of infection by ~ 7% per year – less than the 17% [86] annual decline needed to achieve the target of 90% reduction in syphilis incidence by 2030 [6].
Despite the significant infection burden, STI surveillance and response in MENA continue to be rudimentary [21], and far below the coverage targets of WHO Global Health Sector Strategy for STIs [6]. Infected individuals are often identified through routine case notifications with surveillance/testing being largely limited to HIV [21,24,87], and sexual health programs, where they exist, cater to general population women rather than women at high risk [24].
Although our expansive search identified considerable evidence at the regional-level, including data that will appear in the scientific literature for the first time, evidence varied by country. Over half of countries had no data on any of the STIs in this key population, less than a third had data on C. trachomatis, N. gonorrhoeae, or T. vaginalis, and only three countries had data on HSV-2 IgG (Table 1, Table 2 and Table 3). This outcome is of concern, given the considerable, yet preventable, STI infection burden among FSWs in the region (Table 4), and the major “core group” role that CHSNs play in STI transmission in any population [10]. Indeed, while the population proportion of FSWs (proportion of FSWs out of the total women population) varies across countries and may seem relatively small [18,88], the size of CHSNs is large suggesting a considerable number of women and men at risk of STI-related morbidity, either through engagement in high sexual risk behavior, or through onward infection transmission [89].
Availability of STI data stands in contrast to HIV data, for which the volume of evidence among FSWs was several fold higher and encompassed most countries [18]. Attending to WHO Global Health Sector Strategy on STIs [6] necessitates a major expansion of STI research and surveillance, as has been done for HIV [17,87,90]. Regrettably, integrated bio-behavioural surveillance surveys (IBBSS) among key populations continue to be focused on HIV, rarely incorporating STIs [91,92]. This presents an important, yet lost, opportunity for monitoring STI levels and trends in key populations, informing programming efforts, gaining an in-depth understanding of sexual networks’ structure, and advancing STI research in this region [13,91,93].
Subregion and time explained most variation in syphilis prevalence—each explained over a third of the variation, and both (remarkably) explained ~ 50% of the variation (Table 5). The strong subregional differences, with Horn of Africa showing the highest prevalence, followed by North Africa, and then Eastern MENA (Table 5 and Table S7 in Online Supplementary Document), appear to reflect variability in the risk environment, such as differences in structure of sexual networks [24], condom use [18], and access to care [24]. The same pattern has been seen in HIV epidemiology among FSWs [18].
There was strong evidence for a time trend of decreasing odds of infection at ~ 7% per year (Table 5, and Table S8 and Figure S1 in the OSD), consistent with, but smaller than, the decline reported for the general population in MENA in a recent global analysis [86], and the declines reported for the general populations in other regions [86]. Different factors may have contributed to this trend including safer sex following the HIV epidemic [94], increased condom use to prevent unwanted pregnancy [18], and HIV-related mortality which may have disproportionally affected populations at higher risk of STIs [95]. This may have been also a consequence of a shorter duration of active syphilis infection in FSWs or their sex partners [96,97], possibly because of improvements in syphilis diagnostics and treatment, or because of widespread use of antibiotics (including for non-STI infections, which sometimes may cure concurrent syphilis) [86].
This being said, recent surveillance data seems also to suggest an increase in syphilis incidence and/or prevalence in other sexual networks or in specific settings, such as among MSM [98-100], and even among reproductive-age women in few countries where congenital syphilis appears to be rising [101,102]. Contributors to these trends may include behavioral factors, such as more sexual partners and unprotected sex among MSM, as well as contextual factors, and possibly even biological factors [99,100,102-104].
Prevalence measures for syphilis and for C. trachomatis in FSWs in MENA were comparable to global levels [22,23], but prevalence measures for N. gonorrhoeae and T. vaginalis leaned towards the lower end of the global range [22,23]. Even though the risk environment among FSWs in MENA seems less conducive to STI transmission, as compared to other regions [18], STI prevalence levels are substantial, perhaps affected by poor access to health care and prevention interventions [21,24,105], as well as absence of enabling environments for this vulnerable population, in a context of criminality [106,107] and stigma [108-110].
While interventions aiming at promoting safer sex, such as condom use, and STI etiological diagnosis and treatment, in high risk populations are widely accepted and advocated for [6,111-114], STI syndromic case management and presumptive treatment have been increasingly subject to criticism amid growing concerns about their role in promoting pathogens’ antimicrobial resistance (AMR) [111,115-119]. Indeed, substantial AMR prevalence and multiple drug resistant strains have been found in gonococcal isolates from FSWs in sub-Saharan Africa [120,121] and elsewhere [122]. This suggests that despite the effectiveness of targeted STI treatment services in reducing STI incidence and prevalence, their appropriateness and sustainable implementation will need to be informed by surveillance and monitoring, notably for AMR, and thus may vary across settings [111,122]. This further supports WHO efforts towards building a global business case for accelerating development of STI vaccines as a fundamental solution to STI drug resistance [123-125].
This study is limited by the quantity and quality of available data. STI prevalence among FSWs remains unknown in over half of countries. While there was considerable evidence for syphilis, less evidence was found for C. trachomatis, N. gonorrhoeae, T. vaginalis, and HSV-2, limiting our ability to conduct advanced meta-analytics—meta-regressions were carried out only for syphilis. Though, for syphilis prevalence, the differences between current vs ever (seropositivity using antibody testing) infection, as well as the differences between diagnostics, were consistent with the findings of a large global analysis for the general population [86], the confidence intervals were wide owing to the smaller number of studies (Table 5). Several measures were based on routine data reporting, and did not include sufficient documentation of study methodology. There was also a wide array of diagnostics used for STI ascertainment, which may have affected observed prevalence.
Available studies may not be representative of the wider population of FSWs, or could be subject to biases, such as selection bias or detection bias. Of note, however, that there was no evidence that any of the assessed study-specific quality domains (Tables S5-S6 in Online Supplementary Document), including sampling methodology, response rate, and explicit indication of the assay used for infection ascertainment, had an effect on prevalence in the multivariable meta-regression (Table 5). Despite limitations, our study provided a detailed synthesis of STI epidemiology in FSWs in MENA, in a background of lack of evidence for this region [22,23]. A significant volume of published and unpublished data was identified and analyzed, and for the first time.
In conclusion, STI levels among FSWs are considerable, supporting a key role for CHSNs in STI transmission dynamics in MENA, and highlighting the public health needs of this neglected and vulnerable population. Despite the progress in our epidemiological understanding, major gaps persist, with no evidence being available for over half of MENA countries. With the limited STI surveillance [24,126], and the focus of programmatic response on case management and syndromic approach, rather than being evidence-informed and grounded on etiological studies [24,126], there is a critical need to expand STI surveillance and the broader STI research agenda. STI testing should be part of IBBSS studies, as well as part of voluntary counseling and testing services for HIV [91,93]. Interventions should factor research findings to ensure adequate and efficient resource allocation. Without such expansion of STI efforts, it will not be possible to monitor infection trends, or to inform a public health response that attends to the WHO Global Health Sector Strategy on STIs [6].