Every year, more than 300 000 children are born with neural tube defects (NTD) [1-6]. NTD are a group of congenital abnormalities that still cause hundreds of thousands of deaths in 04 years age group, while similar number of surviving children remain disabled for life [1-6]. One of the Millennium Development Goals initiated by the United Nations was dedicated to reducing global mortality rates of children in this age group. Since 1990, global child mortality has been declining largely due to the focus on communicable diseases, which included the expansion of immunisation programmes, promotion of breastfeeding and increased provision of mosquito bednets in many countries worldwide [2]. This reduction of mortality has led to the neglected causes of child mortality to be exposed, including that of congenital abnormalities [3-6].
NTD are one of the most common presenting birth defects, arising as a result of incomplete closure of the brain or spinal cord in the 3rd and/or 4th week of pregnancy [3]. NTD can be classified as open or closed, depending on whether neural tissues are exposed or covered by skin, respectively. Open NTD are more frequent and include spina bifida, anencephaly and encephalocele. Closed NTD, such as tethered cord syndrome, are less frequent in comparison [4].
The best known risk factor for foetal NTD is maternal folate deficiency, arising from low levels of vitamin B9 (folic acid) [7,8]. Maternal vitamin B12 deficiency has only recently been shown to independently contribute to risk of NTD [9,10]. Additional risk factors for NTD development include a positive family history, smoking and indoor air pollution from coal and biomass heating used predominantly in developing countries [11-15]. Moreover, NTD are related to maternal socioeconomic status, education, area of residence, and maternal nutrient deficiency or obesity [16,17].
NTD can be identified through simple prenatal testing using ultrasound imaging or maternal serum alphafetoprotein (MSAFP) level screening [18]. Abnormal elevation of MSAFP is a relatively specific and sensitive test for detection of NTD [18]. The abnormal presence of acetylcholinesterases (AChE) in amniotic fluid determined through amniocentesis can also be used for screening of NTD. However, a higherthannormal test result is often not diagnostic and further evaluation should always be undertaken [19,20].
Since the discovery of folic acid as an effective intervention for prevention of neural tube defects [21], many countries have recommended folic acid intake before conception and during pregnancy. However, the dramatic 20% decrease in NTD birth burden after mandatory folic acid fortification (FAF) of enriched products in the US in 1998 showed that there may be more practical ways to administer this intervention [22]. Since this example, many countries such as Chile, Saudi Arabia and South Africa have implemented similar measures to staple food [23-25]. Despite folic acid being a wellknown, costeffective intervention, many developing countries continue to have either ineffective or no policy to increase maternal uptake of folic acid to prevent NTD.
The aims and objectives of this systematic review were:
1. To provide an estimate of NTD burden in LMIC by systematically reviewing literature available in public domain;
2. To examine and discuss the significance of these findings and consider clinical and costeffective interventions and health policies with regards to NTD.
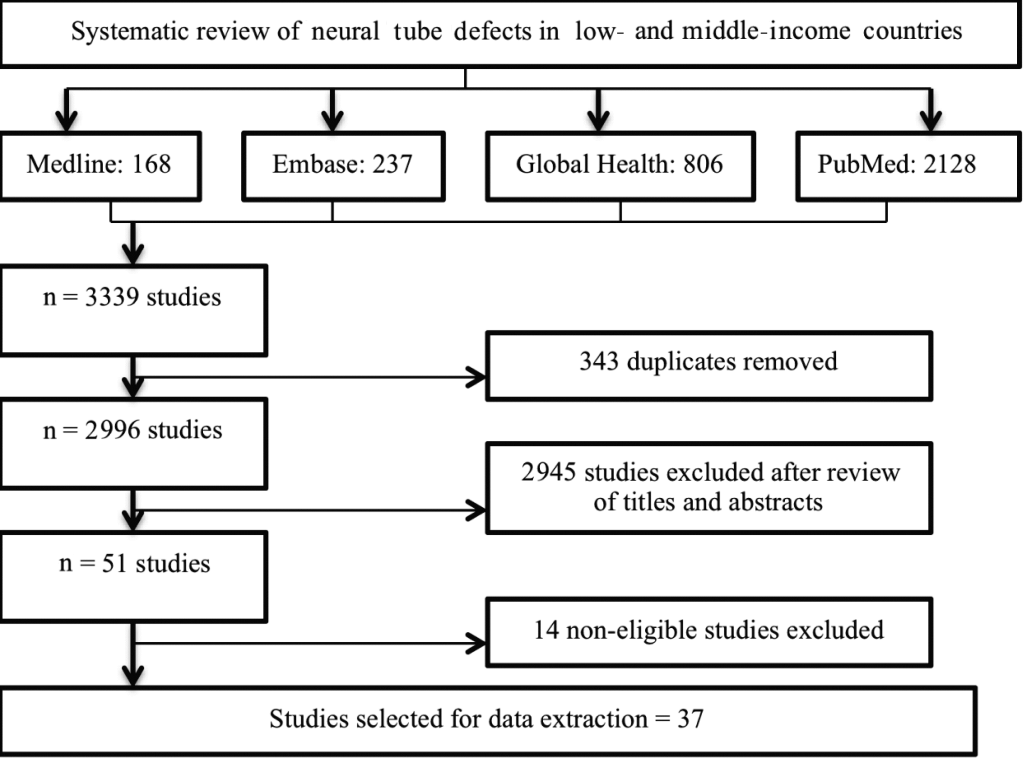
A systematic literature review was conducted to search for published literature regarding populationbased NTD burden estimates in LMIC, through the use of electronic databases: Medline, Embase, Global Health Library and PubMed. Potential further data were searched for on Google Scholar and by crosschecking reference lists from review articles. The search used Medical Subject Headings (MeSH) and key words for the burden of NTD in LMIC, as outlined by the World Bank. Limits of "human" and "2000current" were used to obtain the most up to date NTD burden information. The last searches of the four databases were conducted on 6 February 2013. Search terms for Medline are shown in Table 1 and were modified for other databases as required.
| 1. | exp Developing Countries/ |
| 2. | Developing countr*.tw |
| 3. | (developing adj3 countr*).tw |
| 4. | africa/ or africa, northern/ or algeria/ or egypt/ or libya/ or morocco/ or tunisia/ or "africa south of the sahara"/ or africa, central/ or cameroon/ or central african republic/ or chad/ or congo/ or "democratic republic of the congo"/ or equatorial guinea/ or gabon/ or africa, eastern/ or burundi/ or djibouti/ or eritrea/ or ethiopia/ or kenya/ or rwanda/ or somalia/ or sudan/ or tanzania/ or uganda/ or africa, southern/ or angola/ or botswana/ or lesotho/ or malawi/ or mozambique/ or namibia/ or south africa/ or swaziland/ or zambia/ or zimbabwe/ or benin/ or burkinafaso/ or cape verde/ or cote d'ivoire/ or gambia/ or ghana/ or guinea/ or guineabissau/ or liberia/ or mali/ or mauritania/ or niger/ or nigeria/ or senegal/ or sierra leone/ or togo/ or americas/ or caribbean region/ or west indies/ or "antigua and barbuda"/ or bahamas/ or barbados/ or cuba/ or dominica/ or dominican republic/ or grenada/ or guadeloupe/ or haiti/ or jamaica/ or martinique/ or netherlandsantilles/ or puertorico/ or "saint kitts and nevis"/ or saint lucia/ or "saint vincent and the grenadines"/ or "trinidad and tobago"/ or central america/ or belize/ or costa rica/ or el salvador/ or guatemala/ or honduras/ or nicaragua/ or panama/ or panama canal zone/ or latinamerica/ or mexico/ or south america/ or argentina/ or bolivia/ or brazil/ or chile/ or colombia/ or ecuador/ or frenchguiana/ or guyana/ or paraguay/ or peru/ or suriname/ or uruguay/ or venezuela/ or kazakhstan/ or kyrgyzstan/ or tajikistan/ or turkmenistan/ or uzbekistan/ or borneo/ or brunei/ or cambodia/ or east timor/ or indonesia/ or laos/ or malaysia/ or mekong valley/ or myanmar/ or philippines/ or thailand/ or vietnam/ or bangladesh/ or bhutan/ or india/ or sikkim/ or middle east/ or afghanistan/ or iran/ or iraq/ or israel/ or jordan/ or lebanon/ or saudiarabia/ or syria/ or turkey/ or united arab emirates/ or yemen/ or nepal/ or pakistan/ or srilanka/ or far east/ or china/ or tibet/ or "democratic people's republic of korea"/ or mongolia/ or taiwan/ or albania/ or lithuania/ or bosniaherzegovina/ or bulgaria/ or "macedonia (republic)"/ or moldova/ or montenegro/ or romania/ or russia/ or bashkiria/ or dagestan/ or moscow/ or siberia/ or serbia/ or ukraine/ or armenia/ or atlantic islands/ or azores/ azerbaijan/ or "georgia (republic)"/ or comoros/ or madagascar/ or mauritius/ or seychelles/ or vanuatu/ or micronesia/ or palau/ or expsamoa/ or americansamoa/ or "independent state of samoa"/ or tonga/ |
| 5. | exp neural tube defects/ or anencephaly/ or arnoldchiari malformation/ or encephalocele/ or meningocele/ or meningomyelocele/ or "pentalogy of cantrell"/ or exp spinal dysraphism/ or nervous system malformation |
| 6. | "neural tube defect".tw |
| 7. | "neural tube defects".tw |
| 8. | NTD.tw |
| 9. | exp Prevalence/ |
| 10. | prevalen*.tw |
| 11. | 1 or 2 or 3 or 4 |
| 12. | 5 or 6 or 7 or 8 |
| 13. | 9 or 10 |
| 14. | 11 and 12 and 13 |
| 15. | Limit 14 to (humans and yr = "2000 Current") |
Study selection
The inclusion criteria for relevant papers included population or hospital based studies conducted in LMIC, which were geographically defined taking into account both the World Health Organization's and the World Bank's classification and treating any discrepancies in an inclusive, rather than exclusive way. The studies needed to have clearly expressed NTD burden showing a denominator, published between 2000 and 2013. The searches were limited to the period after the year 2000 in order to generate an estimate that is reflective of reasonably recent NTD trends. No limit on language and publication type was set. Keeping in mind that many babies with NTD are stillborn or terminated through miscarriages and abortions, we decided to include studies with live births, stillbirths and terminations as a separate body of evidence, in addition to studies that used live birthsbased denominators to report the burden of NTD.
Studies conducted solely in specialist hospital units were excluded, as they are likely to report a burden enriched for severe cases that would not be representative of the general population. Studies with incomplete data or where NTD burden could not be calculated were also excluded.
Data extraction
For the 37 retained studies, relevant data were extracted and compiled into Microsoft Excel spreadsheets. Data including authors, country, study size and diagnostic criteria for specific NTD type and total NTD cases were extracted. Types of NTD included spina bifida, myelomeningocele, meningocele, anencephaly, encephalocele and "other NTD types". Burden was expressed using the number of cases observed and a total sample of live births (or, alternatively, a total sample of live births, stillbirths and terminations).
Data analysis
When the number of affected children was not specifically provided in the study, it was calculated with the sample population using the following equation:
Estimated NTD burden = Number of observed NTD cases/Sample size (eg, number of live births) × 1000
The estimates of the burden provided in the retained studies were separated into two categories: those in which the denominator was based on the number of live births, and the other group in which live births, stillbirths and terminations were all included. Wherever this information was available for both categories, figures were separately added to the respective groups. The median estimate of the NTD burden and the interquartile range (IQR) for all LMIC regions was then determined, based on the retained 37 studies. Eventually, the median was multiplied by the number of livebirths in LMIC in the year 2010, according to UN Population Division's estimates (www.un.org/esa/population/), to determine the absolute number of NTD cases that has been introduced to the LMIC in 2010.
A review of relevant databases performed independently by two researchers (AL and SS) identified a total of 3339 studies, but only 37 satisfied all criteria for inclusion (as shown in Figure 1 ). Of the retained studies, 20 reported NTD rates in live births only, 14 reported rates in live births, stillbirths and terminations combined, and 3 studies reported both.
The median sample size from all reviewed papers was 36 331, which corresponded well to a typical study size. The median sample population in studies based on live births was 35 974, compared to 49 534 in studies based on live births, stillbirths and terminations.
The search retrieved NTD burden from 18 countries in 6 WHO regions ( Table 2 ). The overall burden calculated using the median from studies based on live births was 1.67/1000 (IQR = 0.983.49) for total NTD burden ( Table 3 and 4 ), 1.13/1000 (IQR = 0.751.73) for spina bifida ( Table 3 and 5 ), 0.25/1000 (IQR = 0.081.07) for anencephaly ( Table 3 and 6 ) and 0.15/1000 (IQR = 0.080.23) for encephalocele ( Table 3 and 7 ).Corresponding estimates based on all pregnancies resulting in live births, still births and terminations were 2.55/1000 (IQR = 1.563.91) for total NTD burden ( Table 3 and 8 ), 1.04/1000 (IQR = 0.672.48) for spina bifida ( Table 3 and 9 ), 1.03/1000 (IQR = 0.671.60) for anencephaly ( Table 3 and 10 ) and 0.21 (IQR = 0.160.28) for encephalocele ( Table 3 and 11 ). This translates into about 190 000 neonates who are born each year with NTD in LMICs.
| Number of studies | ||||
|---|---|---|---|---|
| WHO region | Country | Total studies | Live births only | Live births, stillbirths & terminations |
| Western Pacific | China | 6 | 1 | 5 |
| Malaysia | 1 | 0 | 1 | |
| South East Asia | India | 3 | 2 | 1 |
| Pakistan | 1 | 1 | 0 | |
| Thailand | 1 | 1 | 0 | |
| Eastern Mediterranean | Jordan | 2 | 2 | 0 |
| Saudi Arabia | 2 | 2 | 0 | |
| Iran | 4 | 1 | 3 | |
| Europe | Azerbaijan | 1 | 0 | 1 |
| Russia | 1 | 1 | 0 | |
| Ukraine | 1 | 0 | 1 | |
| Turkey | 3 | 2 | 1 | |
| Africa | Cameroon | 1 | 1 | 0 |
| South Africa | 1 | 1 | 0 | |
| Americas | Brazil | 3 | 2 | 1 |
| Colombia | 1 | 1 | 0 | |
| Peru | 1 | 1 | 0 | |
| Chile | 4 | 1 | 3 | |
| Studied outcome | Denominator | Number of Studies | Median (per 1000) | Interquartile range (per 1000) | Minimum(per 1000) | Maximum (per 1000) |
|---|---|---|---|---|---|---|
| All neural tube defects | LB | 23 | 1.67 | 0.983.49 | 0.50 | 12.41 |
| LB+SB+TP | 17 | 2.55 | 1.563.91 | 0.86 | 19.94 | |
| Spina bifida | LB | 14 | 1.13 | 0.751.73 | 0.38 | 5.90 |
| LB+SB+TP | 15 | 1.04 | 0.672.48 | 0.35 | 5.81 | |
| Anencephaly | LB | 13 | 0.25 | 0.081.07 | 0.01 | 11.33 |
| LB+SB+TP | 16 | 1.03 | 0.671.60 | 0.30 | 8.26 | |
| Encephalocele | LB | 9 | 0.15 | 0.080.23 | 0.03 | 0.39 |
| LB+SB+TP | 13 | 0.21 | 0.160.28 | 0.07 | 2.65 |
LB live births; LB+SB+TP live births, stillbirths and terminated pregnancies
| Author and reference | Sample size | Cases | Rate (per 1000 live births) |
|---|---|---|---|
| Amarin et al. [26] | 61 447 | 16 | 0.95 |
| Aqrabawi [11] | 5088 | 33 | 6.50 |
| Asindi et al. [27] | 82 176 | 64 | 0.78 |
| Bademci et al. [28] | 5499 | 17 | 3.09 |
| Behrooz et al. [29] | 13 262 | 56 | 4.22 |
| Chen et al. [30] | 26 599 | 48 | 1.80 |
| Cherian et al. [31] | 1218 | 10 | 8.21 |
| Cortes et al. [32] | 59 627 | 67 | 1.12 |
| Costa et al. [33] | 9386 | 11 | 1.17 |
| Gu et al. [13] | 6420 | 25 | 3.89 |
| Hertrampf et al. [34] | 117 740 | 114 | 0.97 |
| Kaur et al. [35] | 7400 | 5 | 0.68 |
| Khattak et al. [36] | 5560 | 69 | 12.41 |
| Mandiracioglu et al. [37] | 36 331 | 56 | 1.54 |
| Njamnshi et al. [38] | 52 710 | 98 | 1.86 |
| Pachajoa et al. [39] | 32 995 | 55 | 1.67 |
| Petrova et al. [40] | 141 159 | 298 | 2.11 |
| Pacheco et al. [41] | 24 964 | 124 | 4.97 |
| Ricks et al. [42] | 35 974 | 72 | 2.00 |
| Safdar et al. [24] | 33 489 | 42 | 1.25 |
| Sayed et al. [25] | 46 021 | 45 | 0.98 |
| Wasant et al. [43] | 180 000 | 114 | 0.63 |
| Yuskiv et al. [33] | 75 609 | 38 | 0.50 |
| Author and reference | Sample size | Cases | Rate(per 1000 live births) |
|---|---|---|---|
| Aqrabawi[11] | 5088 | 30 | 5.90 |
| Asindi et al. [27] | 82 176 | 46 | 0.56 |
| Bademci et al. [28] | 5499 | 11 | 2.00 |
| Behrooz et al. [29] | 13 262 | 23 | 1.73 |
| Cherian et al. [31] | 1218 | 6 | 4.93 |
| Costa et al. [44] | 9386 | 7 | 0.75 |
| Khattak et al. [36] | 5560 | 5 | 0.90 |
| Mandiracioglu et al. [37] | 36 331 | 43 | 1.18 |
| Njamnshi et al. [38] | 52 710 | 65 | 1.23 |
| Petrova et al. [40] | 141 159 | 147 | 1.04 |
| Ricks et al. [42] | 35 974 | 62 | 1.72 |
| Safdar et al. [24] | 33 489 | 36 | 1.07 |
| Sayed et al. [25] | 46 021 | 25 | 0.54 |
| Yuskiv et al. [33] | 75 609 | 29 | 0.38 |
| Author and reference | Sample size | Cases | Rate (per 1000 live births) |
|---|---|---|---|
| Asindi et al. [27] | 82 176 | 3 | 0.04 |
| Behrooz et al. [29] | 13 262 | 30 | 2.26 |
| Cherian et al. [31] | 1218 | 3 | 2.46 |
| Costa et al. [44] | 9386 | 1 | 0.11 |
| Khattak et al. [36] | 5560 | 63 | 11.33 |
| Mandiracioglu et al. [45] | 36 331 | 4 | 0.11 |
| Njamnshi et al. [38] | 52 710 | 4 | 0.08 |
| Petrova et al. [40] | 141 159 | 151 | 1.07 |
| Ricks et al. [42] | 35 974 | 10 | 0.28 |
| Safdar et al. [24] | 33 489 | 1 | 0.03 |
| Sayed et al. [25] | 46 021 | 17 | 0.37 |
| Wasant et al. [43] | 180 000 | 45 | 0.25 |
| Yuskiv et al. [33] | 75 609 | 1 | 0.01 |
| Author and reference | Sample size | Cases | Rate (per 1000 live births) |
|---|---|---|---|
| Aqrabawi [11] | 5088 | 2 | 0.39 |
| Asindi et al. [27] | 82 176 | 15 | 0.18 |
| Behrooz et al. [29] | 13 262 | 3 | 0.23 |
| Costa et al. [44] | 9386 | 3 | 0.32 |
| Mandiracioglu et al. [45] | 36 331 | 1 | 0.03 |
| Njamnshi et al. [38] | 52 710 | 5 | 0.09 |
| Safdar et al. [24] | 33 489 | 5 | 0.15 |
| Wasant et al. [43] | 180 000 | 14 | 0.08 |
| Yuskiv et al. [33] | 75 609 | 3 | 0.03 |
| Author and reference | Sample size | Cases | Rate (per 1000 live births stillbirths and terminations) |
|---|---|---|---|
| Aguiar et al. [46] | 18 807 | 89 | 4.73 |
| Cortes et al. [32] | 60 072 | 94 | 1.56 |
| Cortes et al. [12] | 486 779 | 419 | 0.86 |
| Dai et al. [46] | 2 281 616 | 2873 | 1.30 |
| Golalipour et al. [47] | 37 951 | 109 | 2.87 |
| Golalipour et al. [12] | 30 639 | 78 | 2.55 |
| Golalipour et al. [48] | 49 534 | 194 | 3.91 |
| Gu et al. [13] | 6420 | 128 | 19.94 |
| Li et al. [10] | 11 534 | 159 | 13.79 |
| Liu et al. [49] | 99 888 | 122 | 1.22 |
| Mahadevan et al. [45] | 54 738 | 310 | 5.66 |
| Nazer et al. [50] | 434 624 | 740 | 1.70 |
| Noraihan et al. [51] | 34 109 | 37 | 1.08 |
| Onrat et al. [52] | 8631 | 31 | 3.59 |
| Rad et al. [53] | 14 121 | 117 | 2.57 |
| Yuskiv et al. [33] | 75 928 | 159 | 2.09 |
| Zhang et al. [54] | 62 373 | 126 | 2.02 |
| Author and reference | Sample size | Cases | Rate (per 1000 live births, stillbirths and terminations) |
|---|---|---|---|
| Cortes et al. [32] | 60 072 | 46 | 0.77 |
| Cortes et al. [12] | 486 779 | 204 | 0.42 |
| Golalipour et al. [47] | 37 951 | 62 | 1.63 |
| Golalipour et al. [12] | 30 639 | 39 | 1.27 |
| Gu et al. [13] | 6420 | 25 | 3.89 |
| Li et al. [10] | 11 534 | 67 | 5.81 |
| Liu et al. [49] | 99 888 | 59 | 0.59 |
| Mahadevan et al. [45] | 54 738 | 170 | 3.11 |
| Noraihan et al. [51] | 34 109 | 12 | 0.35 |
| Onrat et al. [52] | 8631 | 9 | 1.04 |
| Yuskiv et al. [33] | 75 928 | 64 | 0.84 |
| Aguiar et al. [55] | 18 807 | 47 | 2.49 |
| Dai et al. [46] | 2 281 616 | 1369 | 0.60 |
| Nazer et al. [50] | 434 624 | 374 | 0.86 |
| Rad et al. [53] | 14 121 | 35 | 2.48 |
| Author and reference | Sample size | Cases | Rate (per 1000 live births, stillbirths and terminations) |
|---|---|---|---|
| Aguiar et al. [55] | 18 807 | 24 | 1.28 |
| Cortes et al. [32] | 60 072 | 37 | 0.62 |
| Cortes et al. [12] | 486 779 | 147 | 0.30 |
| Dai et al. [46] | 2 281 616 | 1140 | 0.50 |
| Golalipour et al. [47] | 37 951 | 43 | 1.40 |
| Golalipour et al. [12] | 30 639 | 35 | 0.92 |
| Golalipour et al. [48] | 49 534 | 56 | 1.13 |
| Gu et al. [13] | 6420 | 53 | 8.26 |
| Li et al. [10] | 11 534 | 75 | 6.50 |
| Liu et al. [49] | 99 888 | 42 | 0.42 |
| Mahadevan et al. [45] | 54 738 | 98 | 1.80 |
| Nazer et al. [50] | 434 624 | 311 | 0.72 |
| Noraihan et al. [51] | 34 109 | 25 | 0.73 |
| Onrat et al. [52] | 8631 | 12 | 1.39 |
| Rad et al. [53] | 14 121 | 78 | 5.52 |
| Yuskiv et al. [33] | 75 928 | 62 | 0.82 |
| Author and reference | Sample size | Cases | Rate (per 1000 live births stillbirths and terminations) |
|---|---|---|---|
| Aguiar et al. [55] | 18 807 | 5 | 0.27 |
| Cortes et al. [32] | 60 072 | 11 | 0.18 |
| Dai et al. [46] | 2 281 616 | 365 | 0.16 |
| Golalipour et al. [47] | 37 951 | 4 | 0.11 |
| Golalipour et al. [12] | 30 639 | 4 | 0.13 |
| Gu et al. [13] | 6420 | 17 | 2.65 |
| Li et al. [10] | 11 534 | 17 | 1.47 |
| Liu et al. [49] | 99 888 | 7 | 0.07 |
| Mahadevan et al. [45] | 54 738 | 36 | 0.66 |
| Nazer et al. [50] | 434 624 | 91 | 0.21 |
| Onrat et al. [52] | 8631 | 2 | 0.23 |
| Rad et al. [53] | 14 121 | 4 | 0.28 |
| Yuskiv et al. [33] | 75 928 | 12 | 0.16 |
As expected, when comparing IQRs as the robust predictions, overall NTD burden estimates were found to be higher in the live births, stillbirths and terminations group in comparison to studies that included only live births, while spina bifida was the most commonly reported NTD type. Moreover, there is internal consistency in the presented estimates, because the sum of the specific NTD types always fits into the "envelope" of all NTD.
This systematic literature review aimed to examine the burden of NTD in LMIC. It is, to our knowledge, the first study to quantitatively estimate the total NTD burden in LMIC. As such, the burden estimates can be successfully used in the muchneeded preventive policy development in LMIC with high risk of NTD.
The results from the 37 selected studies [10-13,23-55] suggest that NTD burden is approximately twice as high, if not higher, in LMIC than in highincome countries [56-58]. The findings from live birthonly studies showed that the median total NTD burden is 1.67 per 1000 live births, although there were reports of significantly higher values, with a maximum burden as high as 12.41/1000. The overall median is greater in studies where live births, stillbirths and terminations were taken into account, where the burden is 2.55 per 1000 and maximum reported burden of 19.94/1000. This is expected, as a considerable proportion of NTD result in stillbirths and terminations [59,60].
Significant discrepancies between reported burdens from the same country were sometimes observed. These differences were attributed to different study settings, for example in rural and urban India [31,35,45], or different time periods as seen in two studies from Jordan [11,26]. Extremely high burden of NTD of 13.79 and 19.94 was observed in two studies from China, although the samples were rather small, indicating a possible selection bias [10,13].
Regardless of the progress in control of NTDs observed in highincome countries, NTD continue to be a problem of significant public health impact in LMIC. NTD have detrimental physical and emotional effects on the affected children and their caregivers, and may present a lifelong important and often insurmountable economic problem, especially to poor families [52]. The cost of raising a child with spina bifida from birth to 18 years of age in Chile was estimated to be around US$ 120 000 [34]. These expenses, apart from causing individual deprivation, are a significant economic burden on the level of the whole society, causing a vicious circle of poverty in the LMIC.
Hundreds of thousands of live born babies are affected by NTD in LMIC, which remain an important and preventable cause of morbidity and mortality. Thus, effective policies for prevention are vital to reduce the burden of NTD on individuals and on society. Up to now, more than 59 countries have committed to mandatory fortification programmes [59,61,62]. However, many LMIC still have ineffective recommendations and policies towards folic acid uptake. Some countries have recommended the improvement of daily diet and folic acid supplement use, but do not have a mandatory policy [59,61,62]. Recommendation provides a good starting point for reducing NTD burden in LMIC. However, many households in LMIC may not be able to afford folic acid supplementation throughout pregnancy [63]. As shown by the example from the US where NTD burden had fallen by 20% after mandatory fortification, recommendation alone, even without the economic constraint, is not likely to provide a feasible and effective solution [22].Interestingly, survey conducted in the UK found that there was only a marginal increase in folic acid intake in women who were planning pregnancy [64]. Additionally, around half of all pregnancies in the US are unexpected [58,62], and this figure may be even higher in LMIC where there may be limited availability of contraception.
Despite obvious benefits, before promoting folic acid fortification, many factors must be considered. Currently no country in the European Union has compulsory fortification schemes due to risk consideration and campaigns against 'mass medication' [65,66]. Safety, ethics and economic feasibility of a FAF programme must be taken into account before implementing such a policy, especially on a wholecountry level. Nevertheless, current high burden of NTD in LMIC stresses the need for a comprehensive prevention program.
For consistent and reliable estimates on burden of NTD, it is important to set up vital and birth registration documentation programs in countries that lack coherent information on NTD burden. Not only will this aid in the prevention and treatment of NTD, but it will also enable policy makers to monitor the benefits of implemented prevention programs. This may be particularly important for countries in the African WHO region, where a high NTD burden is expected, but from which only a few studies have been published [67,68].
The reported NTD burden was estimated based on a limited number of available studies, some with very variable sample sizes that differed in inclusion of stillbirths and terminated births in the study design. We could not use metaanalysis, because studies came from such heterogeneous contexts that we didn't feel it was justified to present anything beyond simple median and IQR in this initial estimate. This is partly because not all studies adhered to ICD10 classification of NTD and were not uniformly conducted regarding method of diagnosis and reporting of NTD type, enabling potential over or underestimation of NTD burden through misdiagnosis. Also, the technical restrictions of accounting for all stillbirths and terminations in the examined studies limited the precision of our estimated burden in that population [68].
Finally, the data was available from studies conducted in only 18 countries, implying that the studied sample is unlikely to be representative of all the LMIC globally. Regardless of these significant limitations, it is our opinion that the estimated burdens reported in the results provide useful data for initial assessment of NTD burden in LMIC. An increase in high quality research on NTD, especially with regards to gender and geographical regions, should be prioritised to allow more accurate NTD estimates. This would make the burden of the problem easier to estimate in a more credible way, and allow effective planning of prevention and intervention to minimise the risks for NTD.