|
|
|
William Mude, Victor M Oguoma, Tafadzwa Nyanhanda, Lillian Mwanri, and Carolyne Njue
|
Abstract
Background
People from racial minority groups in western countries experience disproportionate socioeconomic
and structural determinants of health disadvantages. These disadvantages have led to
inequalities and inequities in health care access and poorer health outcomes. We report
disproportionate disparities in prevalence, hospitalisation, and deaths from COVID-19 by racial
minority populations.
Methods
We conducted a systematic literature search of relevant databases to identify studies reporting
on prevalence, hospitalisations, and deaths from COVID-19 by race groups between 01 January 2020
– 15 April 2021. We grouped race categories into Blacks, Hispanics, Whites and Others.
Random effects model using the method of DerSimonian and Laird were fitted, and forest plot with
respective ratio estimates and 95% confidence interval (CI) for each race category, and subgroup
meta-regression analyses and the overall pooled ratio estimates for prevalence, hospitalisation
and mortality rate were presented.
Results
Blacks experienced significantly higher burden of COVID-19: prevalence ratio 1.79 (95% confidence
interval (CI) = 1.59-1.99), hospitalisation ratio 1.87 (95%
CI = 1.69-2.04), mortality ratio 1.68 (95% CI = 1.52-1.83), compared
to Whites: prevalence ratio 0.70 (95% CI = 0.0.64-0.77), hospitalisation ratio
0.74 (95% CI = 0.65-0.82), mortality ratio 0.82 (95%
CI = 0.78-0.87). Also, Hispanics experienced a higher burden: prevalence ratio
1.78 (95% CI = 1.63-1.94), hospitalisation ratio 1.32 (95%
CI = 1.08-1.55), mortality ratio 0.94 (95% CI = 0.84-1.04) compared
to Whites. A higher burden was also observed for Other race groups: prevalence ratio 1.43 (95%
CI = 1.19-1.67), hospitalisation ratio 1.12 (95% CI = 0.89-1.35),
mortality ratio 1.06 (95% CI = 0.89-1.23) compared to Whites. The disproportionate
burden among Blacks and Hispanics remained following correction for publication bias.
Conclusions
Blacks and Hispanics have been disproportionately affected by COVID-19. This is deeply concerning
and highlights the systemically entrenched disadvantages (social, economic, and political)
experienced by racial minorities in western countries; and this study underscores the need to
address inequities in these communities to improve overall health outcomes.
|
In December 2019, a new pneumonia-like infection with varying symptoms, ranging from mild to severe shortness
of breath, emerged from Wuhan, China [1]. A World Health Organisation
(WHO) investigation designated the infection as a 2019 novel coronavirus and was subsequently named COVID-19
[2]. The infection quickly spread throughout the world; at the time of
writing, the source has not yet been determined. It was declared a public health emergency of international
concern by WHO in January 2020 and became a pandemic in March [3,4]. Transmission occurs through air droplets, and no proven cure had
existed against the virus. As of 24 May 2021 at 2:50 pm Central European Summer Time (CEST), there
have been 166 860 081 confirmed COVID-19 infection cases and 3 459 996 related
deaths worldwide and rising [5].
Available evidence suggests that medical comorbidities, obesity, diabetes, old age, and being a male are risk
factors for COVID-19 [6,7].
However, in countries where data has been reported for race, the data shows that the burdens of COVID-19 are
disproportionately high among racial minority groups [6,8]. For example, in the United Kingdom, the United States of America,
and Brazil, high cases of COVID-19 are reported in people from racial minority groups [9-12]. The reports show that in the United
Kingdom and the United States of America, 35% and 33% of COVID-19 patients, respectively, are from racial
minority populations [7,10],
although these populations make much lower proportions of the total population. Some experts have claimed
these disproportionately high burdens of COVID-19 are a result of health disparities and entrenched
inequities experienced by minority communities [13]. However,
conflicting findings relating to the reported burden of COVID-19 by racial groups have shown different
rates. Some reports have claimed that White populations have higher mortality than Blacks, Asian, and
Minority Ethnic (BAME) groups, while others showed that BAME groups have higher cases or no differences [
14-16]. A recently published
systematic review by Pan and colleagues [17] suggests that Blacks
have a high risk of acquiring COVID-19 infection and worse clinical outcomes than Whites. However, the
systematic review conducted by Pan and colleagues [17] did not run a
meta-analysis and only summarised finding from each of the studies without synthesising them to highlight
the extent of the issue.
In this article, we report our systematic review and meta-analysis of the literature on the burden of
COVID-19 by race. We assessed the disparity of COVID-19 prevalence, hospitalisation and mortality ratios
among Blacks, Hispanics, Whites, and Other race groups. This review provides vital information on the
burdens of COVID-19 among the selected race group and will help support policies that address health
inequities.
METHODS
Search strategy
Our search strategy was guided by the PRISMA statement [18].
We conducted the systematic search strategy in the following databases and conference proceedings:
CINAHL Complete, Medline, Web of Science (Science Citation Index Expanded (SCI-EXPANDED), Social
Sciences Citation Index (SSCI), Arts & Humanities Citation Index (A&HCI), Conference
Proceedings Citation Index- Science (CPCI-S), Conference Proceedings Citation Index- Social Science
& Humanities (CPCI-SSH), and Emerging Sources Citation Index (ESCI)) for peer-reviewed papers
published between 1 January 2020 – 15 April 2021. We searched these databases and conference
proceedings using the search terms: TI(prevalence OR incidence OR burden* OR rate* OR death* OR
Mortali*) AND (COVID-19 OR 2019 novel coronavirus disease OR COVID19 OR COVID-19 pandemic OR
SARS-CoV-2 infection OR COVID-19 virus disease OR 2019 novel coronavirus infection OR 2019-nCoV
infection OR coronavirus disease 2019 OR coronavirus disease-19 OR 2019-nCoV disease OR COVID-19
virus infection) AND (people of colo* OR minor* OR immigrant* OR African American OR Hispanics OR
black* OR emigrant* OR ethnic). We also searched grey literature and government websites for
COVID-19 data by race.
Outcomes
The primary outcomes assessed were COVID-19 prevalence, hospitalisations, and deaths by the following
selected race categories: Blacks, Hispanics, Whites, and Other race groups. Blacks were defined as
people with African ancestral origins who self-identify or are identified as Black, African or
Afro-Caribbean [19,20
]. Whites were described as people with European ancestral origins who identify or are identified as
White non-Hispanic [20]. Hispanics were defined as people of
Spanish speaking backgrounds from Central and South America who identify or are identified by others
as Hispanic or Latino. Other race groups were defined as race groups not identified as Whites,
Hispanics or Blacks (Asians, Indigenous, mixed-race, and unknown). Few COVID-19 studies have
categorised Asians as a separate racial group when examining COVID-19 outcomes by race. Because of
this, we have grouped Asian in “Other race groups”.
Screening and appraisal
The lead author, the third co-author and the last co-author conducted the search and screening
process separately, identifying studies by titles and abstracts. Studies that reported race data on
prevalence or deaths or hospitalisations were included in this study. Studies were excluded if they
were commentaries, letters, reviews, and opinions. At the same time, studies that reported
prevalence, deaths, and hospitalisation for other health conditions were excluded. After removing
the duplicates, studies were screened by titles, abstracts and read in full. Studies that met the
inclusion criteria (
Figure 1
) were appraised for quality using the JBI quality appraisal for systematic literature
reviews [21].
Figure 1.
Flow diagram of studies included in the review. 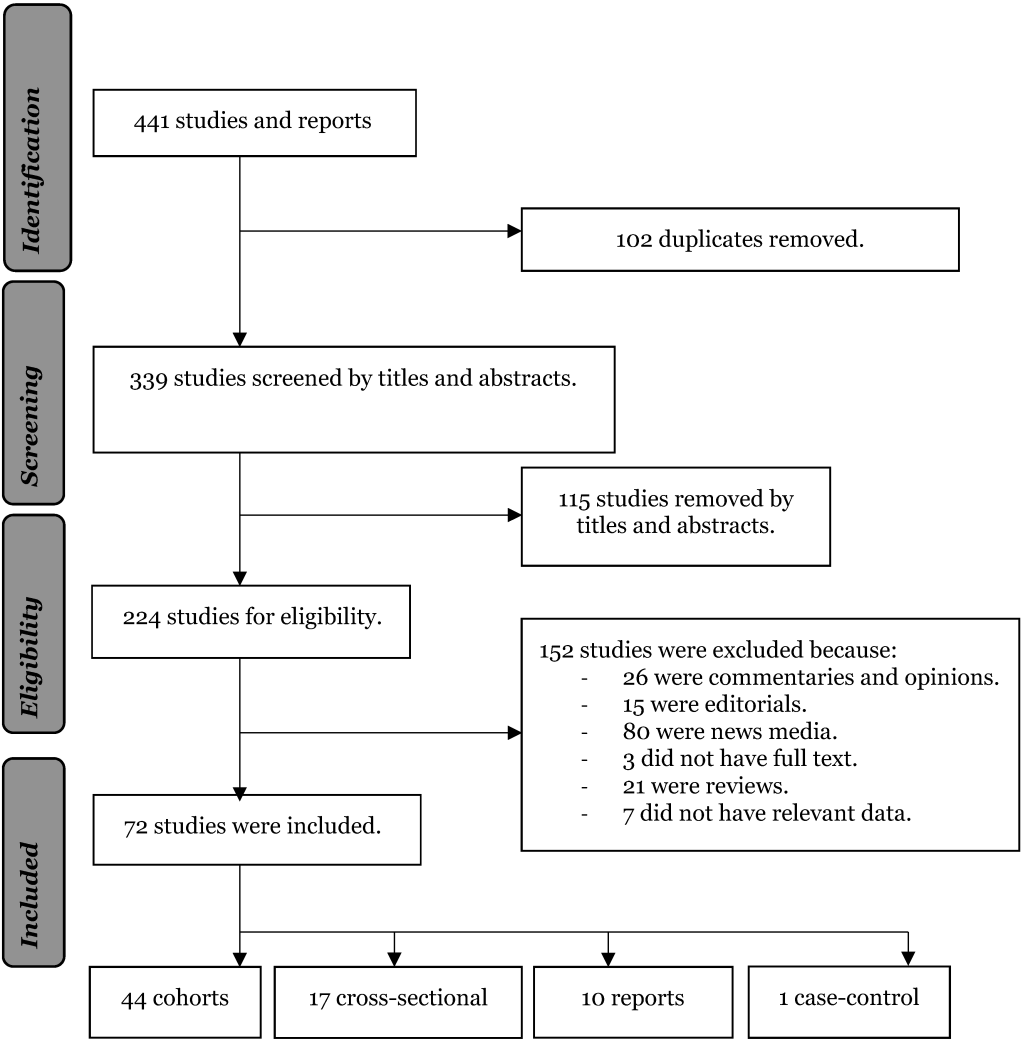 Data extraction and meta-analysis
We independently extracted information from studies that met the inclusion criteria. Information on
the author, year of publication, country, study design, race, population proportion, samples, cases,
prevalence, deaths, and hospitalisation was extracted, where appropriate. We separated the studies
into four different race groups: Blacks, Hispanics, Whites, and Other race groups (Asians,
Indigenous, mixed-race, and others).
We calculated the expected number of cases, hospitalisations, and deaths for each study, where
appropriate. We used the expected number of cases, hospitalisations, and deaths to calculate the
prevalence, hospitalisation, and mortality rate ratios weighted by the population of each race
group. Where population proportion for race category was not provided, we used respective country
census data (USA [22] and UK [23]) to determine each race proportion to establish weighted prevalence ratio,
hospitalisation ratio, and mortality rate.
We assumed that the probability for the occurrence of the expected cases, hospitalisations and deaths
was the same as the probabilities for the occurrence of the observed cases, hospitalisations, and
deaths in the absence of any changes in the rate of infectivity and the risk of exposure. We also
assumed that all members of the population were at risk of infection, hospitalisations, and deaths
from COVID-19 because no vaccination had existed at the time and that the pattern of the outbreak
was random. That is, people randomly took up testing for COVID-19 from drive-by testing centres and
hospitals, and therefore the reported samples were representative of the population group.
We calculated 95% confidence intervals (CI) using both the exact Poisson distribution approach for
observed and expected counts <100 and the approach for observed and expected counts >100 [
24]. Random effects model using DerSimonian and
Laird’s methods were fitted, and forest plot with respective ratio estimates and 95% CI were
presented for each race category in subgroup analyses and the overall pooled ratio estimates for
prevalence, hospitalisation and mortality. The -statistic was used to test the overall effect with
statistical significance set at α≤0.005 Heterogeneity between studies was assessed
using the I2 statistic. Nonparametric trim-and-fill analysis was used to
assess for publication bias [25,26]. Multivariable meta-regression was performed to explore heterogeneity
resulting from the relationship between study effect sizes and race, country, year, and study
design. The metan Stata module in Stata 16.1 MP (StataCorp, College Station, TX, USA) was used to
conduct the meta-analysis [27].
RESULTS
Studies
We included 72 studies that reported at least observed cases, hospitalisations, or deaths from
COVID-19. The included studies and the period covered by each study are provided in Table S1 in the
Online Supplementary Document
. Fifty-four studies (75.0%) were from the United States of America, 13 (18.1%) from the
United Kingdom, and five (6.9%) from Brazil. Forty-four studies were cohorts (61.1%), 10 reports
(13.9%), 17 cross-sectional (23.6%) and one case-control (1.4%). Twelve studies (16.2%) reported the
observed cases, hospitalisations, and deaths [28-40]. Ten studies (13.5%) reported observed hospitalisations and
deaths [41-50], and
eight studies (12.2%) reported cases and deaths [34,51-58]. Two studies
(2.8%) reported observed cases and hospitalisations [59,60]. Seventeen studies (22.6%) reported observed cases only [
61-77]. Twelve studies
(16.2%) reported hospitalisations [78-88], and 11 (14.9%) reported deaths only [12
,89-98].
COVID-19 prevalence ratio
Thirty-nine studies (54.2%) (22 cohorts [28-32,34,36,40,52-57,59,62,63,66,67,73-75], ten cross-sectional [35
,51,58,61,64,65,68-70,72], six reports [
33,38,39,71,76,77], and one
case-control study [60]) had data to calculate the
standardised prevalence ratio of COVID-19 for the identified race categories in the general
community. The pooled prevalence ratio for Blacks was 1.79 (95% CI = 1.59, 1.99;
I2 = 99.9%, P < 0.001) (
Figure 2
, Panel A), Hispanics 1.78 (95% CI = 1.63, 1.94; I2
= 99.9%, P < 0.001) (
Figure 2
, Panel B), Other race groups 1.43 (95% CI = 1.19, 1.67;
I2 = 100.0%, P < 0.001) (
Figure 2
, Panel C), and Whites 0.70 (95% CI = 0.64, 0.77; I2
= 99.9%, P < 0.001) (
Figure 2
, Panel D).
Figure 2.
Standardised prevalence ratio (SPR) Forest Plots of COVID-19 by selected race groups. Panel
A: Blacks. Panel B: Hispanics. Panel C: Other race groups. Panel D: Whites. 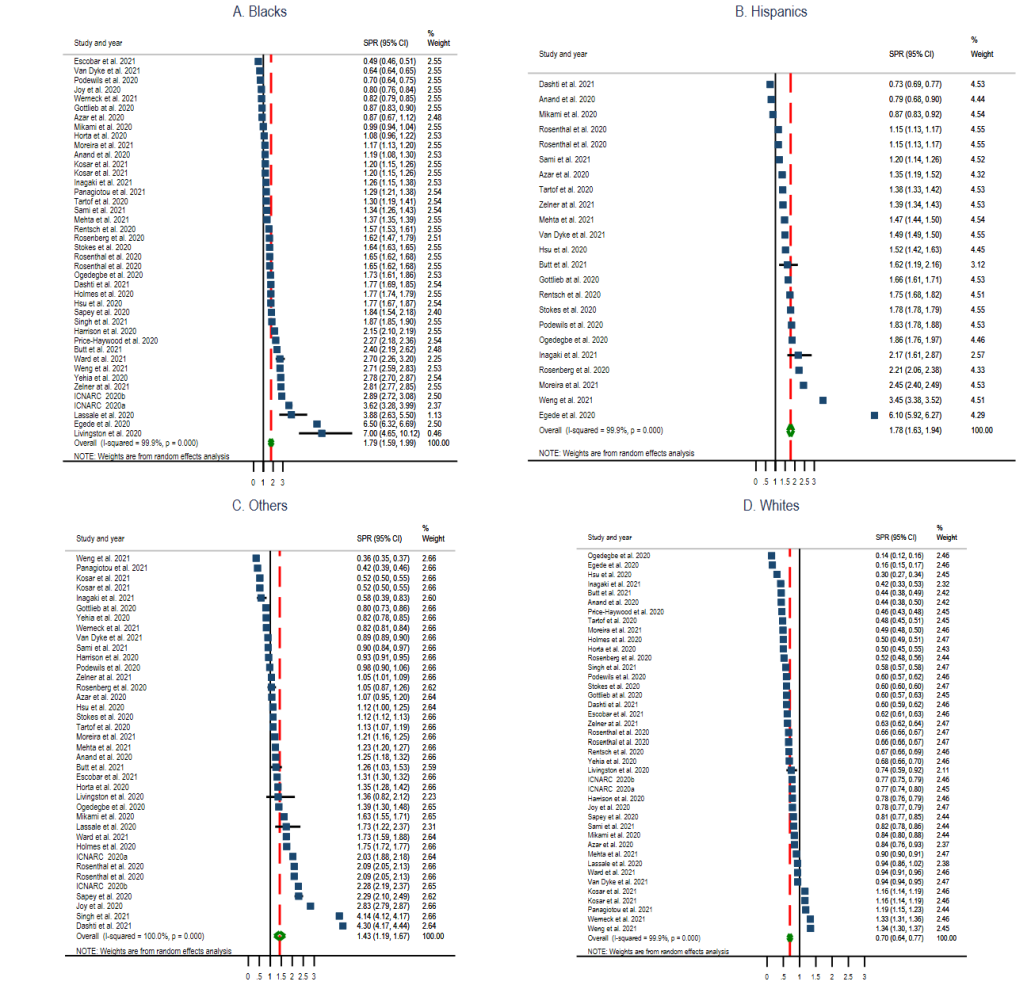 As shown in
Figure 3
, the overall pooled COVID-19 prevalence ratio for all population was 1.36 (95%
CI = 1.29-1.43; I2 = 99.97%, P
< 0.001). Subgroup analysis showed that UK had the highest of COVID-19
prevalence ratio, 1.86 (95% CI = 1.53, 2.19), followed by USA, 1.32 (95%
CI = 1.53, 2.19) and Brazil, 0.92 (95% CI = 0.68, 1.17). The COVID-19
prevalence ratio reported in studies published between the year 2020 and 2021 were 1.40 (95%
CI = 1.30, 1.50) and 1.32 (95% CI = 1.21, 1.42), respectively. Cohort
studies reported the highest COVID-19 prevalence ratio of 1.41 (95% CI = 1.32, 1.51)
followed by reports 1.35 (95% CI = 1.19, 1.52) and cross-sectional studies 1.31 (95%
CI = 1.10, 1.53).
Figure 3.
Standardised prevalence ratio (SPR) Forest plots of COVID-19 by race, country, year, and
study design. 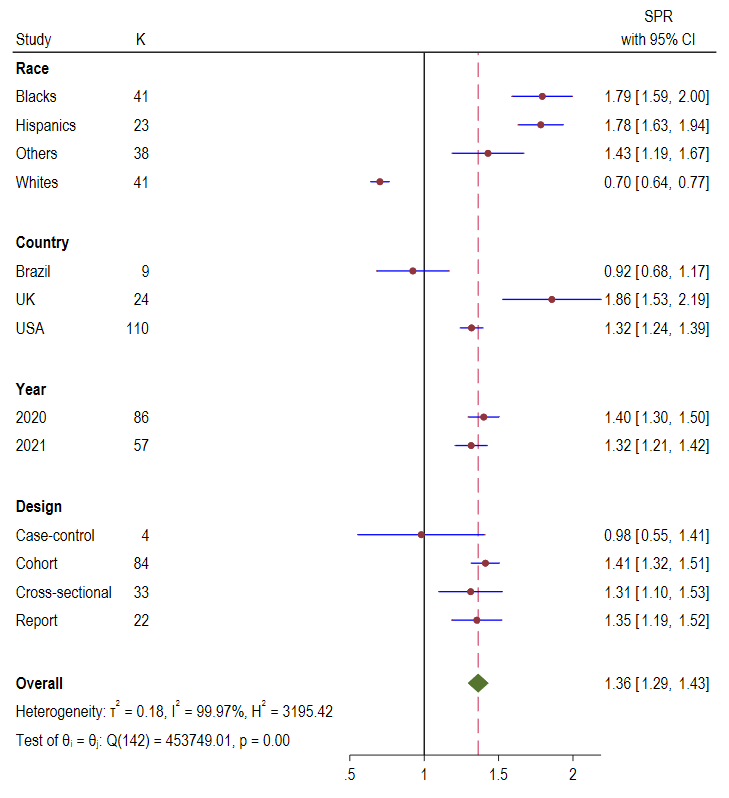 COVID-19 hospitalisation ratio
Thirty-six studies (48.6%) (six reports [7,33,38,39,88], twenty-six
cohorts [16,28-32,36,40,41,43-50,59,69,78-81,84,87,88], three cross-sectional [35
,42,86], and
one case-control [60]) reported data with information on
hospitalisation by race. The pooled estimate of hospitalisation ratio among Blacks was 1.87 (95%
CI = 1.69, 2.04; I2 = 99.5%, P
< 0.001) (
Figure 4
, Panel A), Hispanics 1.32 (95% CI = 1.08, 1.55; I2
= 99.1%, P < 0.001) (
Figure 4
, Panel B), Other race groups 1.12 (95% CI = 0.89, 1.35; I2
= 99.7%, P < 0.00) (
Figure 4
, Panel C), and Whites 0.74 (95% CI = 0.65, 0.82; I2
= 99.7%, P < 0.001) (
Figure 4
, Panel D).
Figure 4.
Standardised hospitalisation ratio (SHR) Forest plots of COVID-19 by selected race groups.
Panel A: Blacks. Panel B: Hispanics. Panel C: Other race groups. Panel D: Whites. 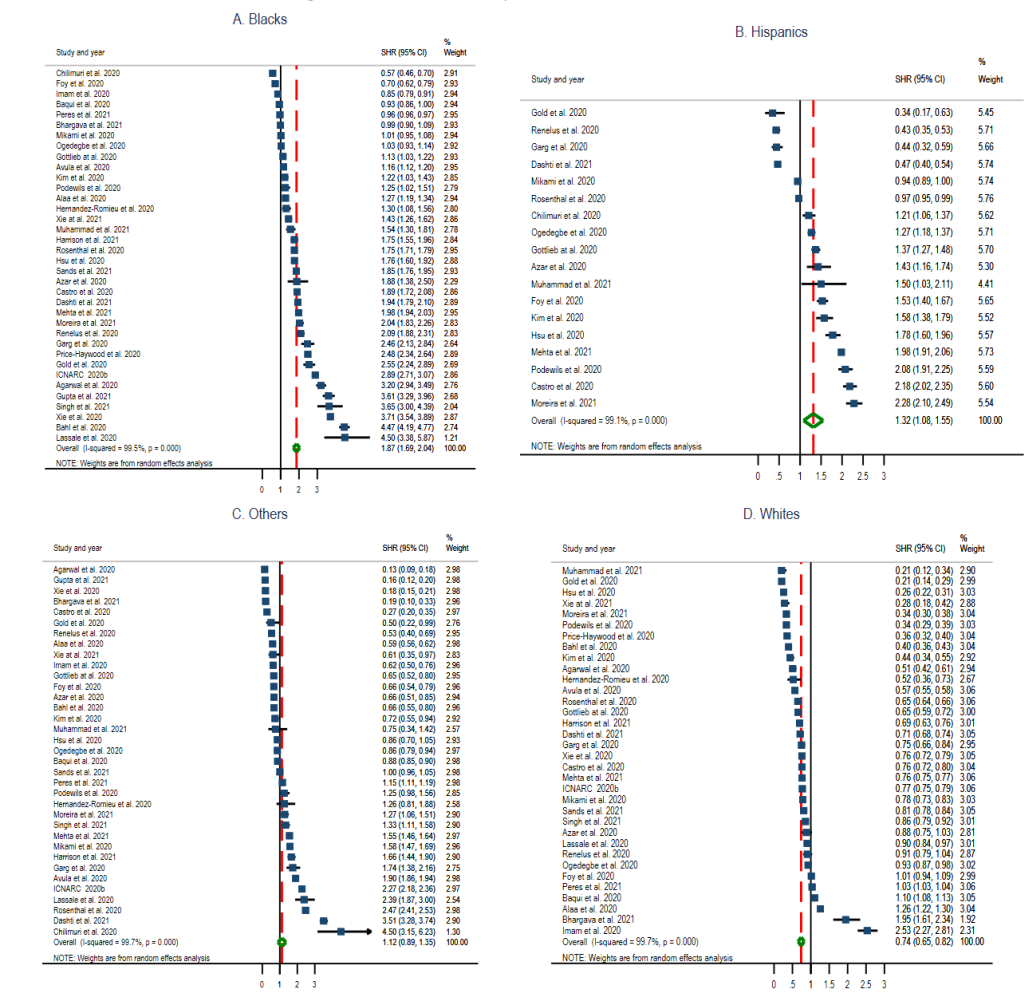 The pooled overall hospitalisation ratio was 1.23 (95% CI = 1.16, 1.29;
I2 = 99.67%, P < 0.001) (
Figure 5
). Country level analysis showed that UK experienced the highest hospitalisation ratio 1.64
(95% CI = 1.38, 1.90) followed by USA 1.22 (95% CI = 1.13, 1.31) and
Brazil 1.01 (95% CI = 0.96, 1.06). Analysis by study designs found that cohort studies
reported the highest hospitalisation ratio, 1.26 (95% CI = 1.18, 1.33), followed by
cross-sectional 1.03 (95% CI = 0.87, 1.18), reports 1.23 (95%
CI = 0.99-1.46) and case-control 0.95 (95% CI = 0.60, 1.31).
Figure 5.
Standardised hospitalisation ratio (SHR) Forest plots of COVID-19 by race, country, year, and
study design. 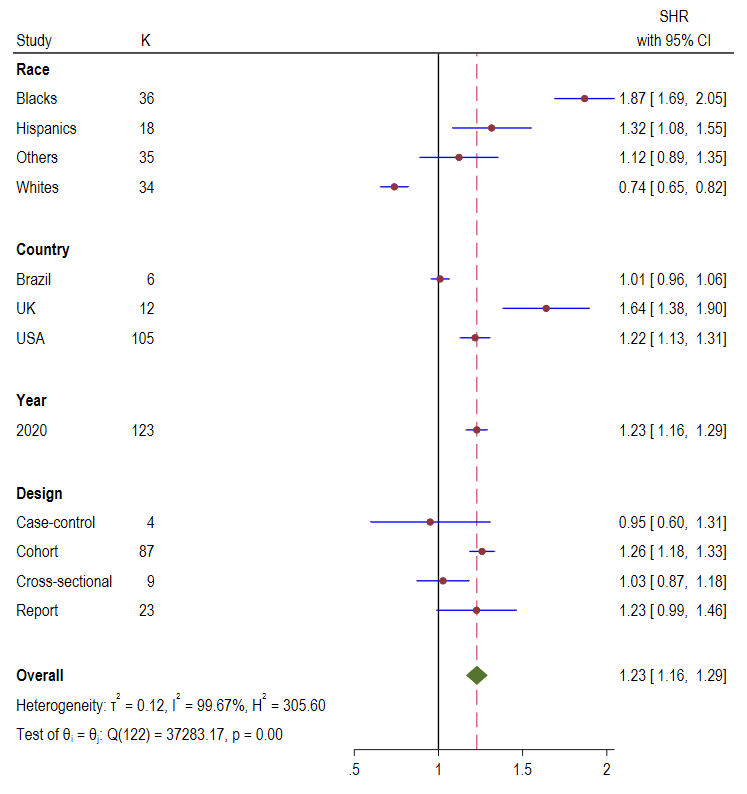 COVID-19 mortality ratio
Forty-two studies (56.8%) (twenty-nine cohorts [12,28-32,34,36,40,41,43-50,52-57,89,90,93,96], four reports [
38,39,41,90] and nine
cross-sectional studies [35,
42,51,58,
91,94,95,97,98]) reported data with information to determine mortality
rates by race groups.
Figure 6
presents the forest plots for mortality ratio by race. The plots showed that the pooled
estimate for the mortality ratio in Blacks was 1.68 (95% CI = 1.52, 1.83;
I2 = 99.5%, P < 0.00) (
Figure 6
, Panel A), Hispanics 0.94 (95% CI = 0.84, 1.05; I2
= 98.2%, P < 0.00) (
Figure 6
, Panel B), Other race groups 1.06 (95% CI = 0.89, 1.23; I2
= 99.8%, P < 0.00) (
Figure 6
, Panel C), and Whites 0.82 (95% CI = 0.78, 0.87; I2
= 99.1%, P < 0.00) (
Figure 6
, Panel D).
Figure 6.
Standardised mortality ratio (SMR) Forest plots of COVID-19 by selected race groups. Panel A:
Blacks. Panel B: Hispanics. Panel C: Other race groups. Panel D: Whites. 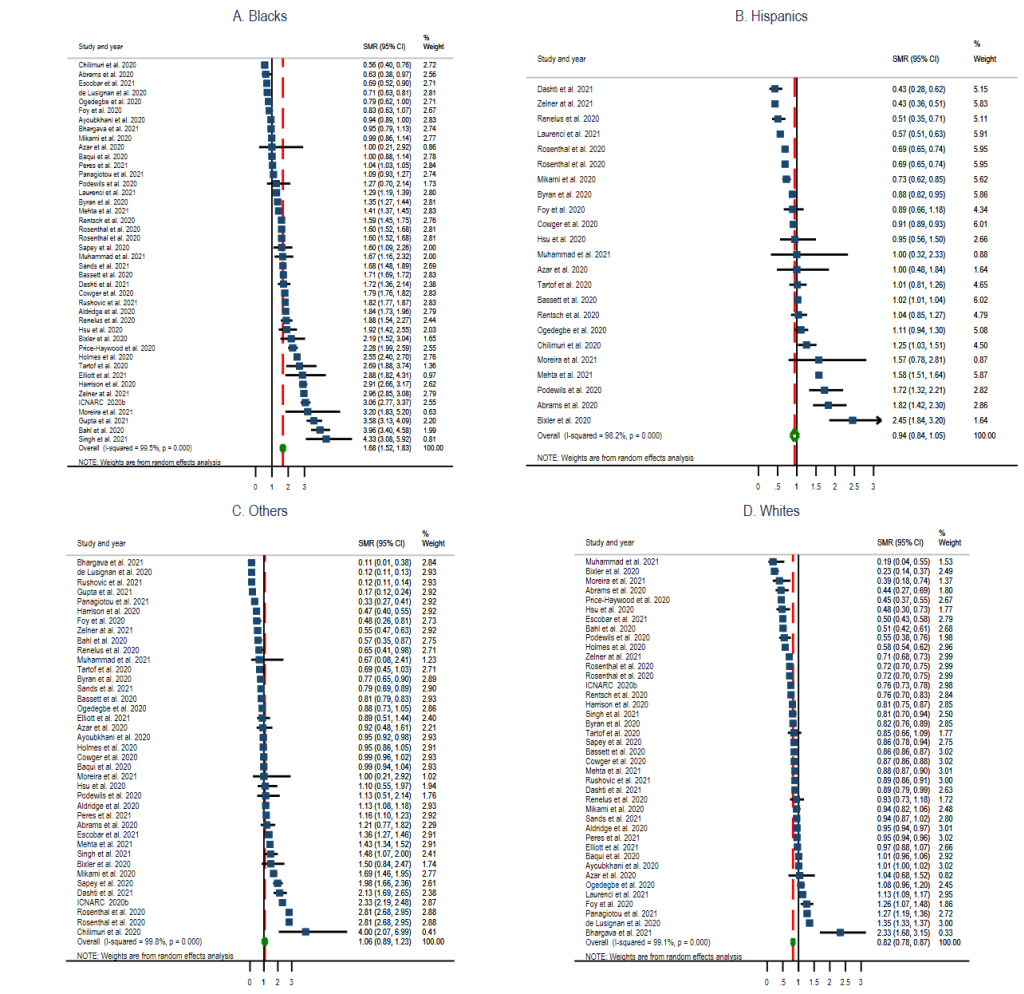 The overall pooled mortality ratio for all population shown in
Figure 7
was 1.13 (95% CI = 1.07, 1.1.20; I2
= 99.75%, P < 0.00). Mortality ratio by country was
highest for UK 1.33 (95% CI = 1.10, 1.57), followed by USA 1.12 (95%
CI = 1.05, 1.19), and Brazil 0.98 (95% CI = 0.98, 1.05). Report studies
had the highest mortality ratio 1.42 (95% CI = 1.03, 1.82), followed by cohort 1.10
(95% CI = 1.05, 1.15), and cross-sectional 1.06 (95% CI = 0.90, 1.22).
Figure 7.
Standardised mortality ratio (SMR) Forest plots of COVID-19 by race, country, year, and study
design. 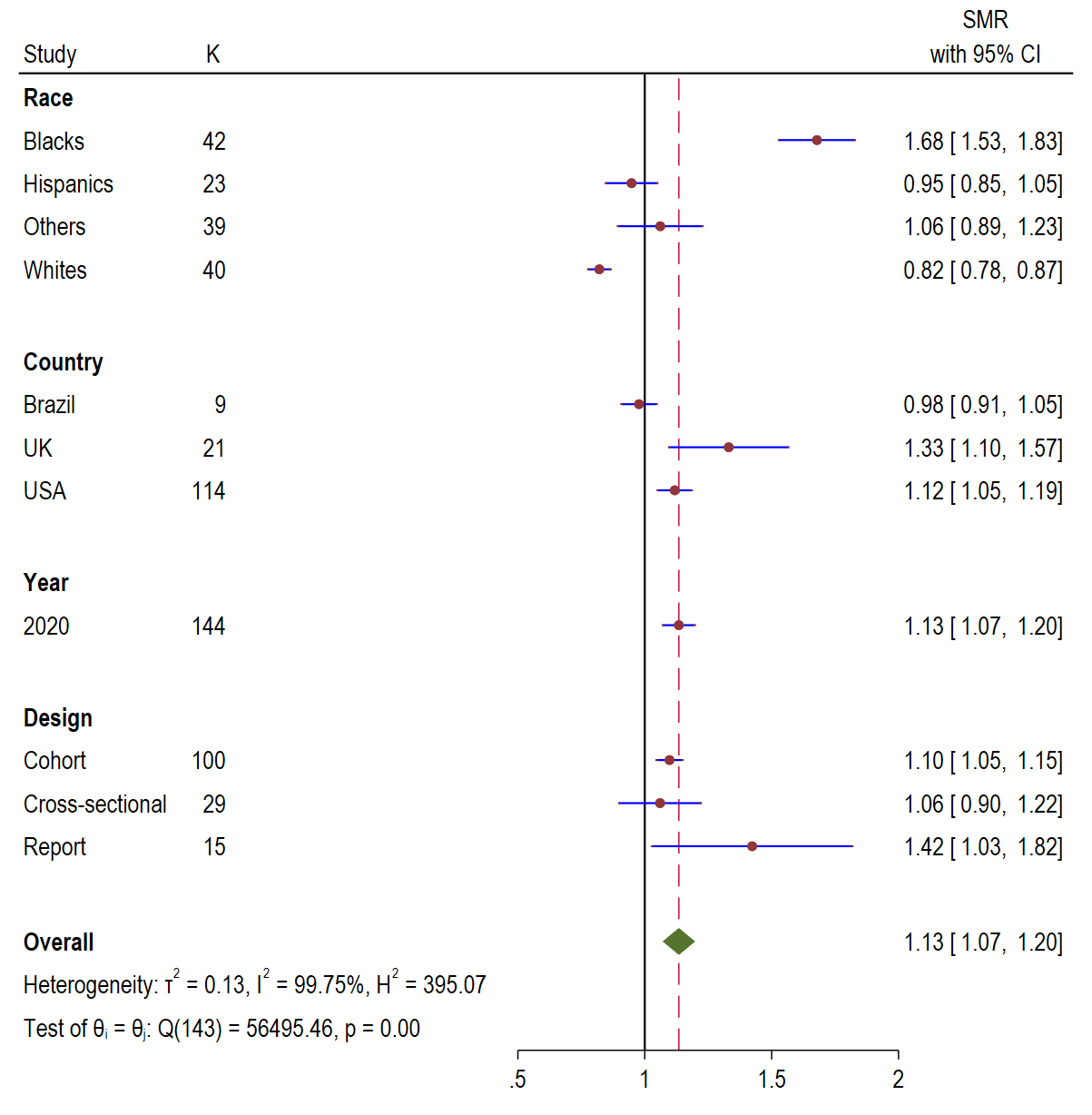 Meta-analysis and regression
Table 1
shows the mean difference in COVID-19 outcomes for prevalence, hospitalisation, and
mortality ratio. It was found that the prevalence ratio between Blacks and Whites was significant,
-1.09 (95% CI = -1.28, -0.90; P < 0.000). A
significant finding was also observed between Blacks and Other race groups, -0.35 (95%
CI = -0.55, -0.16; P < 0.000). However, the
difference in COVID-19 prevalence ratio between Blacks and Hispanics was not significant, 0.11 (95%
CI = -0.12, 0.34; P = 0.34). For the mean difference in
prevalence ratio by study designs, a significant difference was observed only between case-control
and cohort study design, 0.48 (95% CI = 0.04, 0.93; P
= 0.03). No significant mean difference is prevalence ratio was found between the
studies published in 2020 and 2021, -0.07 (95% CI = -0.22, 0.09; P
= 0.39).
Table 1.
Meta-regression of Mean difference for prevalence, hospitalisation, and mortality ratios by
subgroups
| Outcomes |
Parameters |
Mean difference (95% CI) |
P value
|
|
Prevalence ratio
|
| Race
|
Black
|
Ref
|
.
|
|
|
Hispanics
|
0.11 (-0.12, 0.34)
|
0.343
|
|
|
Other
|
-0.35 (-0.55, -0.16)
|
<0.001
|
|
|
White
|
-1.09 (-1.28, -0.90)
|
<0.001
|
| Country
|
Brazil
|
Ref
|
.
|
|
|
UK
|
0.91 (0.56, 1.26)
|
<0.001
|
|
|
USA
|
0.19 (-0.13, 0.51)
|
0.248
|
| Year
|
2020
|
Ref
|
.
|
|
|
2021
|
-0.07 (-0.22, 0.09)
|
0.387
|
| Design
|
Case-control
|
Ref
|
.
|
|
|
Cohort
|
0.48 (0.04, 0.93)
|
0.033
|
|
|
Cross-sectional
|
0.25 (-0.22, 0.72)
|
0.302
|
|
|
Report
|
0.24 (-0.23, 0.71)
|
0.312
|
|
Hospitalisation ratio
|
| Race
|
Black
|
Ref
|
.
|
|
|
Hispanics
|
-0.49 (-0.73, -0.24)
|
<0.001
|
|
|
Other
|
-0.77 (-0.97, -0.56)
|
<0.001
|
|
|
White
|
-1.11 (-1.31, -0.91)
|
<0.001
|
| Country
|
Brazil
|
Ref
|
.
|
|
|
UK
|
0.67 (0.24, 1.09)
|
<0.001
|
|
|
USA
|
0.11 (-0.26, 0.49)
|
0.558
|
| Design
|
Case-control
|
0.00 (0.00, 0.00)
|
.
|
|
|
Cohort
|
0.30 (-0.12, 0.72)
|
0.158
|
|
|
Cross-sectional
|
0.11 (-0.42, 0.63)
|
0.691
|
|
|
Report
|
0.21 (-0.24, 0.65)
|
0.366
|
|
Mortality ratio
|
| Race
|
Black
|
Ref
|
.
|
|
|
Hispanics
|
-0.65 (-0.83, -0.47)
|
<0.001
|
|
|
Other
|
-0.60 (-0.75, -0.44)
|
<0.001
|
|
|
White
|
-0.82 (-0.97, -0.68)
|
0.00
|
| Country
|
Brazil
|
Ref
|
.
|
|
|
UK
|
0.27 (0.01, 0.53)
|
0.039
|
|
|
USA
|
0.13 (-0.10, 0.35)
|
0.276
|
| Design
|
Cohort
|
Ref
|
.
|
|
|
Cross-sectional
|
-0.07 (-0.21, 0.07)
|
0.318
|
|
Report |
0.26 (0.05, 0.46) |
0.014 |
For hospitalisation ratio, it was found that the mean difference in hospitalisation ratio in Blacks
was significantly different from Whites -1.11 (95% CI = -1.31, -0.91; P
< 0.001), Hispanics -0.49 (95% CI = -0.73, -0.24; P
< 0.001), and Other race groups -0.77 (95% CI = -0.97,
-0.56; P < 0.001). Intercountry analysis showed COVID-19 mean
difference in hospitalisation ratio was significant between Brazil and UK 0.67 (0.24, 1.09;
P < 0.001) but not between Brazil and USA 0.11 (95%
CI = -0.26, 0.49; P = 0.560). The mean difference in
hospitalisation ratios between study designs were not significant.
The mean difference in mortality ratio between Blacks and Hispanics -0.65 (95%
CI = -0.83, -0.47; P < 0.00), Blacks and Whites
-0.82 (95% CI = -0.97, -0.68; P < 0.001), Blacks and
Other race groups -0.60 (95% CI = -0.75, -0.44; P
< 0.00) were all significant. Country level analysis showed that the mean
difference between Brazil and UK was significant 0.27 (95% CI = 0.01, 0.53; P
= 0.04), but no difference existed between Brazil and USA 0.13 (95%
CI = -0.10, 0.35; P = 0.28). Analysis for study design
found that the mean difference in mortality ratio between cohorts and reports was significant, 0.26
(95% CI = 0.05, 0.46; P = 0.01) but not between cohorts
and cross-sectional studies, -0.07 (95% CI = -0.21, 0.07; P
= 0.32).
Publication bias
Following correction for publication bias (Appendix S1-S3 and Figures S1-S3 in the
Online Supplementary Document
), the prevalence ratio among Blacks and Hispanics remained high; 1.38 (95%
CI = 1.19, 1.57) for Blacks and 1.59 (95% CI = 1.43, 1.75) for Hispanics
compared to 0.69 (0.62, 0.75) for Whites and 1.01 (95% CI = 0.83, 1.37) for Other race
groups. For hospitalisation ratio, Blacks and Hispanics continued to have a high hospitalisation
ratio even when corrected for publication bias compared to Whites and Other race groups. For
example, the hospitalisation ratio for Black was 1.42 (95% CI = 1.25, 1.59), Hispanics
1.32 (1.08-1.55) compared to 0.67 (95% CI = 0.59, 0.75) for Whites and 0.74 (0.49,
0.98) for Other race groups. The mortality ratio for Blacks remained high, 1.32 (1.17, 1.45),
compared to Hispanics 0.82 (95% CI = 0.70, 0.93), Whites 0.82 (95%
CI = 0.78, 0.87) and Other race groups 0.83 (95% CI = 0.67, 0.99)
following correction for publication bias.
DISCUSSION
The reviewed studies showed that COVID-19 significantly impacted Blacks across all the outcomes measured
compared to Whites. The study found that the prevalence ratios in Blacks were 156% higher than in
Whites, for Hispanics were 154% higher, and for Other race groups were 104% higher. There was a
significant difference between prevalence ratios in Blacks and Whites and Other race groups but not
Hispanics. Hospitalisation ratios in Blacks were 153% higher than in Whites, for Hispanics were 78%
higher, and for Other race groups were 51% higher. A significant difference was found between
hospitalisation in Blacks and Hispanics, Whites and Other race groups. Deaths in Blacks were 105% higher
than in Whites, Hispanics were 15% higher, and Other race groups were 29% higher. Mortality in Blacks
was significantly different from Whites, Hispanics, and Other race groups. Intercountry differences were
also observed regarding prevalence ratios of COVID-19. The prevalence ratio in the USA was 102% higher
than Brazil’s, and UK’s was 43% higher than Brazil’s. Although Blacks and Hispanics
experienced a similar burden of COVID-19, Blacks had higher hospitalisation and mortality ratios than
Hispanics, Whites and Other race groups.
The identified racial disparities in prevalence, hospitalisations and mortality ratio from COVID-19 could
be attributed to several reasons. It could be that Blacks, Hispanics, and Other racial groups experience
higher socioeconomic disadvantages that increase their risk of contracting COVID-19. People in higher
socioeconomic status and affluent neighbourhoods have been reported to be less likely to acquire
COVID-19 infection, whereas social and economic disadvantages have been associated with higher COVID-19
infections [99,100].
Evidence suggests that racial minorities in urban settings tend to live in more crowded conditions and
are more likely to be employed in public-facing occupations (for example, services and transportation),
making practising social distancing practically impossible [13,
15,101]. For example, one
study found that Blacks and Latinos working in essential services experienced higher COVID-19 mortality
rates than Whites [102]. The authors observed that many Blacks
held the top nine essential jobs that exposed them to the risk of catching COVID-19, increasing the
potentials of infecting their families. Another study found that frontline jobs mainly occupied by
Blacks and Hispanics were risk factors to COVID-19 [103].
Despite racial minorities being at increased risk of exposure to the virus, they experience increased
barriers to testing, such as health care access, which contributes to delays in obtaining testing until
they are in a more serious condition resulting in poor health outcomes [101].
Structural racism as a key determinant of population health can also explain the disproportionate burdens
of COVID-19 found among racial minorities across the outcomes measured in the current study [104]. While there are differences between and within countries
relating to the experience of structural racism and health inequities because of differences in policies
and forces that facilitate these issues, these systems of oppression can lead to health inequities
because of experienced disadvantages and lack of opportunities [105
,106].
Blacks, Hispanics, and Other minority racial populations experience disproportionately higher rates of
other underlying health conditions, making them more vulnerable to COVID-19. For example, a report found
that neighbourhoods with higher poverty rates occupied by Blacks and Hispanics experienced
disproportionate diabetes and hypertension comorbidities and higher rates of COVID-19 infections [103]. Other studies have shown that comorbidities such as obesity
and cardiovascular diseases, where Blacks and Others are disproportionately overrepresented, were a risk
factor for COVID-19 related mortality [6,107-109]. It may also be that older
Blacks, Hispanics and Other racial minority groups have higher comorbidities than Whites, increasing
their risks to COVID-19. Older age, regardless of race, has, however, been identified as a risk factor
to COVID-19 [107,110,
111].
Other factors, such as underlying health conditions and old age, could have contributed to the increased
risk of COVID-19 in minority populations [42,60,112,113]. However, many underlying health conditions result from years
of systemic inequities in social determinants of health, including poor housing, lack of employment, low
income, racism, poor neighbourhood, and poor working and living conditions experienced by minority
populations in western countries [112,114-117]. For example, residents in poor
neighbourhoods with a poorly built environment to facilitate physical activities are less likely to
participate in physical activities and are more likely to live sedentary lifestyles [118]. Sedentary lifestyles and lack of physical activities are
risk factors for cardiovascular-related health conditions, including but not limited to hypertension,
diabetes, and obesity [112]. In countries such as the United
States, issues related to structural determinants of health are prevalent and disproportionately
experienced by minority populations, which leads to generational inequities [99,119].
Given these findings, there is a need for a shift of focus from treating comorbidities from clinical
perspectives only to addressing broader socioeconomic and structural determinants of health
disadvantages experienced by most Blacks, Hispanics and Other minority groups in western countries [
120]. Unprecedentedly, COVID-19 has exposed the social
disadvantages that Blacks, Hispanics and Other minority populations continue to experience because of
racism, discrimination, and systemic institutional policy of racial suppression in western countries [
104]. Governments and policymakers have an opportunity to turn
the course of health inequities in minority populations by investing in programs that facilitate equity
in disadvantaged communities for improved health outcomes.
Limitations and strengths
There are a few limitations to consider when interpreting the finding from this article. The included
studies used different study designs and populations that could have influenced the selection of
study participants and protocols, and thus, the findings. Some studies did not clearly describe
whether race was self-reported and could have misclassified the race of some patients, which could
have led to under-or over-reporting of COVID-19 outcomes. The included studies did not have data on
many moderators, and we could not conduct a meta-analysis controlling for them. The different
sources of data used by the different studies (for example, hospital data and sentinel data),
differences in specificity and sensitivity of tests used could have contributed to the high
heterogeneity observed among the studies, and the differences in the ascertainment of death outcomes
are important limitations to note. Lastly, we did not search for all the grey literature, which
could have inadvertently led to the omission of additional potential studies. It is, therefore,
important to interpret our findings with these limitations in mind.
An important strength of this paper is its methodologically rigorous analysis that involved
correcting for publication bias and synthesising the findings to highlight the extent of the
disproportionate burden of COVID-19 in racial minority groups. This means that the findings cannot
be attributed to publication bias which ensures confidence in the finding. Another strength of this
review is its use of meta-regression analysis controlling for the different parameters, and this was
vital to compare the results by sub-groups. Our findings highlight the disproportionate burdens of
COVID-19 outcomes among the selected race groups.
CONCLUSIONS
The burden of COVID-19 in terms of prevalence, hospitalisation, and mortality rate was disproportionately
higher among Blacks and Hispanics compared with Whites. These findings point to the systemic
disadvantages experienced by racial minority populations and highlight the need to address inequities in
these communities by developing programs that improve overall health outcomes. Further work and more
well-designed longitudinal studies are needed to expand the knowledge on racial differences in COVID-19
outcomes and to identify the social determinants of health shaping the disparities in the outcome of
COVID-19 among racial minority populations.
Acknowledgements
Ethics: No ethical approval was required as the study used secondary data from published
articles.
REFERENCES
[1]
Z Wu and JM McGoogan. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19)
Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease
Control and Prevention. JAMA. 2020;323:1239-42. DOI: 10.1001/jama.2020.2648. [PMID:32091533]
[2]
P Boldog, T Tekeli, Z Vizi, A Dénes, FA Bartha, and G Röst. Risk Assessment of Novel Coronavirus COVID-19 Outbreaks Outside China.
J Clin Med. 2020;9:571 DOI: 10.3390/jcm9020571. [PMID:32093043]
[5]
World Health Organization. WHO Coronavirus Disease (COVID-19)
Dashboard. 2021. Available: https://covid19.who.int/. Accessed: 24 May
2021.
[6]
R Franki. Comorbidities increase COVID-19 deaths by a factor of 12: Hispanic, Black, and Native
persons account for a disproportionate share of those infected by the coronavirus.
Neurology Reviews. 2020;28:45
[7]
S Garg, L Kim, M Whitaker, A O’Halloran, C Cummings, and R Holstein. Hospitalization Rates and Characteristics of Patients Hospitalized with
Laboratory-Confirmed Coronavirus Disease 2019 - COVID-NET, 14 States, March 1-30, 2020.
MMWR Morb Mortal Wkly Rep. 2020;69:458-64. DOI: 10.15585/mmwr.mm6915e3. [PMID:32298251]
[8]
DR Holtgrave, MA Barranco, JM Tesoriero, DS Blog, and ES Rosenberg. Assessing racial and ethnic disparities using a COVID-19 outcomes continuum for New
York State. Ann Epidemiol. 2020;48:9-14
. DOI: 10.1016/j.annepidem.2020.06.010. [PMID:32723697]
[9]
K Khunti, AK Singh, M Pareek, and W Hanif. Is ethnicity linked to incidence or outcomes of covid-19? BMJ. 2020;369:m1548 DOI: 10.1136/bmj.m1548. [PMID:32312785]
[10]
M Pareek, MN Bangash, N Pareek, D Pan, S Sze, and JS Minhas. Ethnicity and COVID-19: an urgent public health research priority. Lancet. 2020;395:1421-2. DOI: 10.1016/S0140-6736(20)30922-3. [PMID:32330427]
[11]
AK Okoh, C Sossou, NS Dangayach, S Meledathu, O Phillips, and C Raczek. Coronavirus disease 19 in minority populations of Newark, New Jersey. Int J Equity Health. 2020;19:93 DOI: 10.1186/s12939-020-01208-1. [PMID:32522191]
[12]
RW Aldridge, D Lewer, S Katikireddi, R Mathur, N Pathak, and R Burns. Black, Asian and Minority Ethnic groups in England are at increased risk of death
from COVID-19: indirect standardisation of NHS mortality data. Wellcome Open Res. 2020;5:88 DOI: 10.12688/wellcomeopenres.15922.2. [PMID:32613083]
[13]
M Webb Hooper, AM Nápoles, and EJ Pérez-Stable. COVID-19 and Racial/Ethnic Disparities. JAMA. 2020;323:2466-7. DOI: 10.1001/jama.2020.8598. [PMID:32391864]
[14]
Ravi K. Ethnic disparities in COVID-19 mortality: are
comorbidities to blame? In: Cockerham WC, Cockerham GB, editors. The COVID-19 Reader: The Science
and What it Says About The Social. 1 st Edition. ed. Philadelphia, Pennsylvania: Routledge; 2020.
[17]
D Pan, S Sze, JS Minhas, MN Bangash, N Pareek, and P Divall. The impact of ethnicity on clinical outcomes in COVID-19: A systematic review.
EClinicalMedicine. 2020;23:100404
. DOI: 10.1016/j.eclinm.2020.100404. [PMID:32632416]
[18]
D Moher, A Liberati, J Tetzlaff, and DG Altman. The PG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The
PRISMA Statement. PLoS Med. 2009;6:e1000097
. DOI: 10.1371/journal.pmed.1000097. [PMID:19621072]
[19]
C Agyemang, R Bhopal, and M Bruijnzeels. Negro, Black, Black African, African Caribbean, African American or what? Labelling
African origin populations in the health arena in the 21st century. J Epidemiol Community Health. 2005;59:1014
DOI: 10.1136/jech.2005.035964. [PMID:16286485]
[20]
R Bhopal. Glossary of terms relating to ethnicity and race: for reflection and debate.
J Epidemiol Community Health. 2004;58:441
DOI: 10.1136/jech.2003.013466. [PMID:15143107]
[24]
Breslow N, Day N. Statistical Methods in Cancer Research: Volume
II - The Design and Analysis of Cohort Studies. Lyon: International Agency for Research on Cancer;
1987.
[25]
S Duval and R Tweedie. Trim and Fill: A Simple Funnel-Plot–Based Method of Testing and Adjusting for
Publication Bias in Meta-Analysis. Biometrics. 2000;56:455-63
. DOI: 10.1111/j.0006-341X.2000.00455.x. [PMID:10877304]
[26]
S Duval and RA Tweedie. Nonparametric “Trim and Fill” Method of Accounting for Publication Bias
in Meta-Analysis. J Am Stat Assoc. 2000;95:89-98
.
[27]
Harris R, Bradburn M, Deeks J, Harbord R, Altman DG, Steichen T,
et al. METAN: Stata module for fixed and random effects meta-analysis, Statistical Software
Components S456798. 2006. Available: https://ideas.repec.org/s/boc/bocode.html
. Accessed: 15 August 2020.
[28]
H Dashti, EC Roche, DW Bates, S Mora, and O Demler. SARS2 simplified scores to estimate risk of hospitalization and death among patients
with COVID-19. Sci Rep. 2021;11:4945 DOI: 10.1038/s41598-021-84603-0. [PMID:33654180]
[29]
HB Mehta, S Li, and JS Goodwin. Risk Factors Associated With SARS-CoV-2 Infections, Hospitalization, and Mortality
Among US Nursing Home Residents. JAMA Netw Open. 2021;4:e216315
. DOI: 10.1001/jamanetworkopen.2021.6315. [PMID:33787905]
[30]
T Mikami, H Miyashita, T Yamada, M Harrington, D Steinberg, and A Dunn. Risk Factors for Mortality in Patients with COVID-19 in New York City.
J Gen Intern Med. 2021;36:17-26
. DOI: 10.1007/s11606-020-05983-z. [PMID:32607928]
[31]
A Moreira, K Chorath, K Rajasekaran, F Burmeister, M Ahmed, and A Moreira. Demographic predictors of hospitalization and mortality in US children with COVID-19.
Eur J Pediatr. 2021;180:1659-63
. DOI: 10.1007/s00431-021-03955-x. [PMID:33474580]
[32]
G Ogedegbe, J Ravenell, S Adhikari, M Butler, T Cook, and F Francois. Assessment of Racial/Ethnic Disparities in Hospitalization and Mortality in Patients
With COVID-19 in New York City. JAMA Netw Open. 2020;3:e2026881
. DOI: 10.1001/jamanetworkopen.2020.26881. [PMID:33275153]
[33]
LJ Podewils, TL Burket, C Mettenbrink, A Steiner, A Seidel, and K Scott. Disproportionate Incidence of COVID-19 Infection, Hospitalizations, and Deaths Among
Persons Identifying as Hispanic or Latino - Denver, Colorado March-October 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1812
-6. DOI: 10.15585/mmwr.mm6948a3. [PMID:33270613]
[34]
N Rosenthal, Z Cao, J Gundrum, J Sianis, and S Safo. Risk Factors Associated With In-Hospital Mortality in a US National Sample of
Patients With COVID-19. JAMA Netw Open. 2020;3:e2029058
. DOI: 10.1001/jamanetworkopen.2020.29058. [PMID:33301018]
[35]
BM Singh, J Bateman, A Viswanath, V Klaire, S Mahmud, and A Nevill. Risk of COVID-19 hospital admission and COVID-19 mortality during the first COVID-19
wave with a special emphasis on ethnic minorities: an observational study of a single, deprived,
multiethnic UK health economy. BMJ Open. 2021;11:e046556
. DOI: 10.1136/bmjopen-2020-046556. [PMID:33597146]
[36]
KM Azar, Z Shen, RJ Romanelli, SH Lockhart, K Smits, and S Robinson. Disparities In Outcomes Among COVID-19 Patients In A Large Health Care System In
California. Health Aff (Millwood). 2020;39:1253-62. DOI: 10.1377/hlthaff.2020.00598. [PMID:32437224]
[37]
P Hsu and DE Hayes-Bautista. The Epidemiology of Diversity: COVID-19 Case Rate Patterns in California.
J Immigr Minor Health. 2021;23:1-6. [PMID:33620661]
[38]
ICNARC. ICNARC report on COVID-19 in critical care - 07
September 2020. London: Intensive care national audit and research centre (ICNARC); 2020.
[39]
HE Hsu, EM Ashe, M Silverstein, M Hofman, SJ Lange, and H Razzaghi. Race/Ethnicity, Underlying Medical Conditions, Homelessness, and Hospitalization
Status of Adult Patients with COVID-19 at an Urban Safety-Net Medical Center—Boston,
Massachusetts, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:864-9. DOI: 10.15585/mmwr.mm6927a3. [PMID:32644981]
[40]
EG Price-Haywood, J Burton, D Fort, and L Seoane. Hospitalization and Mortality among Black Patients and White Patients with Covid-19.
N Engl J Med. 2020;382:2534-43
. DOI: 10.1056/NEJMsa2011686. [PMID:32459916]
[41]
A Bahl, MN Van Baalen, L Ortiz, NW Chen, C Todd, and M Milad. Early predictors of in-hospital mortality in patients with COVID-19 in a large
American cohort. Intern Emerg Med. 2020;15:1485-
99. DOI: 10.1007/s11739-020-02509-7. [PMID:32970246]
[42]
P Baqui, I Bica, V Marra, and A Ercole. Schaar Mvd. Ethnic and regional variations in hospital mortality from COVID-19 in
Brazil: a cross-sectional observational study. Lancet Glob Health. 2020;8:e1018-26. DOI: 10.1016/S2214-109X(20)30285-0. [PMID:32622400]
[43]
A Bhargava, SM Szpunar, M Sharma, EA Fukushima, S Hoshi, and M Levine. Clinical Features and Risk Factors for In-Hospital Mortality From COVID-19 Infection
at a Tertiary Care Medical Center, at the Onset of the US COVID-19 Pandemic. J Intensive Care Med. 2021;36:711-8. DOI: 10.1177/08850666211001799. [PMID:33759606]
[44]
S Chilimuri, S Haozhe, A Alemam, N Mantri, E Shehi, and J Tejada. Predictors of Mortality in Adults Admitted with COVID-19: Retrospective Cohort Study
from New York City. West J Emerg Med. 2020;21:779-
84. DOI: 10.5811/westjem.2020.6.47919. [PMID:32726241]
[45]
BH Foy, JCT Carlson, E Reinertsen, I Padros, R Valls, R Pallares Lopez, and E Palanques-Tost. Association of Red Blood Cell Distribution Width With Mortality Risk in Hospitalized
Adults With SARS-CoV-2 Infection. JAMA Netw Open. 2020;3:e2022058
. DOI: 10.1001/jamanetworkopen.2020.22058. [PMID:32965501]
[46]
R Gupta, R Agrawal, Z Bukhari, A Jabbar, D Wang, and J Diks. Higher comorbidities and early death in hospitalized African-American patients with
Covid-19. BMC Infect Dis. 2021;21:78 DOI: 10.1186/s12879-021-05782-9. [PMID:33461499]
[47]
R Muhammad, R Ogunti, B Ahmed, M Munawar, S Donaldson, and M Sumon. Clinical Characteristics and Predictors of Mortality in Minority Patients
Hospitalized with COVID-19 Infection. J Racial Ethn Health Disparities. 2021;Online ahead of print
DOI: 10.1007/s40615-020-00961-x. [PMID:33538998]
[48]
IT Peres, LSL Bastos, JGM Gelli, JF Marchesi, LF Dantas, and BBP Antunes. Sociodemographic factors associated with COVID-19 in-hospital mortality in Brazil.
Public Health. 2021;192:15-20
. DOI: 10.1016/j.puhe.2021.01.005. [PMID:33607516]
[49]
BD Renelus, NC Khoury, K Chandrasekaran, E Bekele, WM Briggs, and A Ivanov. Racial Disparities in COVID-19 Hospitalization and In-hospital Mortality at the
Height of the New York City Pandemic. J Racial Ethn Health Disparities. 2020;Online ahead of print
DOI: 10.1007/s40615-020-00872-x. [PMID:32946070]
[50]
KE Sands, RP Wenzel, LE McLean, KM Korwek, JD Roach, and KM Miller. Patient characteristics and admitting vital signs associated with coronavirus disease
2019 (COVID-19)–related mortality among patients admitted with noncritical illness.
Infect Control Hosp Epidemiol. 2020;42:399
-405. DOI: 10.1017/ice.2020.461. [PMID:32928319]
[51]
AL Escobar, TDM Rodriguez, and JC Monteiro. Lethality and characteristics of deaths due to COVID-19 in Rondonia: an observational
study. Epidemiol Serv Saude. 2021;30:e2020763
. DOI: 10.1590/s1679-49742021000100019. [PMID:33331602]
[52]
SL Harrison, E Fazio-Eynullayeva, DA Lane, P Underhill, and GYH Lip. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United
States: A federated electronic medical record analysis. PLoS Med. 2020;17:e1003321
. DOI: 10.1371/journal.pmed.1003321. [PMID:32911500]
[53]
OA Panagiotou, CM Kosar, EM White, LE Bantis, X Yang, and CM Santostefano. Risk Factors Associated With All-Cause 30-Day Mortality in Nursing Home Residents
With COVID-19. JAMA Intern Med. 2021;181:439-
48. DOI: 10.1001/jamainternmed.2020.7968. [PMID:33394006]
[54]
E Sapey, S Gallier, C Mainey, P Nightingale, D McNulty, and H Crothers. Ethnicity and risk of death in patients hospitalised for COVID-19 infection in the
UK: an observational cohort study in an urban catchment area. BMJ Open Respir Res. 2020;7:e000644
. DOI: 10.1136/bmjresp-2020-000644. [PMID:32873607]
[55]
SY Tartof, Q Lei, V Hong, W Rong, RF Nadjafi, and H Fischer. Obesity and Mortality Among Patients Diagnosed With COVID-19: Results From an
Integrated Health Care Organization. Ann Intern Med. 2020;173:773-81
. DOI: 10.7326/M20-3742. [PMID:32783686]
[56]
J Zelner, R Trangucci, R Naraharisetti, A Cao, R Malosh, and K Broen. Racial Disparities in Coronavirus Disease 2019 (COVID-19) Mortality Are Driven by
Unequal Infection Risks. Clin Infect Dis. 2021;72:e88-95
. DOI: 10.1093/cid/ciaa1723. [PMID:33221832]
[57]
CT Rentsch, F Kidwai-Khan, JP Tate, LS Park, JT King, and M Skanderson. Patterns of COVID-19 testing and mortality by race and ethnicity among United States
veterans: A nationwide cohort study. PLoS Med. 2020;17:e1003379
. DOI: 10.1371/journal.pmed.1003379. [PMID:32960880]
[58]
L Holmes, M Enwere, J Williams, B Ogundele, P Chavan, and T Piccoli. Black-white risk differentials in COVID-19 (SARS-CoV2) transmission, mortality and
case fatality in the United States: translational epidemiologic perspective and challenges.
Int J Environ Res Public Health. 2020;17:4322
DOI: 10.3390/ijerph17124322. [PMID:32560363]
[59]
C Lassale, B Gaye, M Hamer, CR Gale, and GD Batty. Ethnic disparities in hospitalisation for COVID-19 in England: The role of
socioeconomic factors, mental health, and inflammatory and pro-inflammatory factors in a
community-based cohort study. Brain Behav Immun. 2020;88:44-9
. DOI: 10.1016/j.bbi.2020.05.074. [PMID:32497776]
[60]
M Gottlieb, S Sansom, C Frankenberger, E Ward, and B Hota. Clinical Course and Factors Associated With Hospitalization and Critical Illness
Among COVID-19 Patients in Chicago, Illinois. Acad Emerg Med. 2020;27:963-73
. DOI: 10.1111/acem.14104. [PMID:32762106]
[61]
S Anand, M Montez-Rath, J Han, J Bozeman, R Kerschmann, and P Beyer. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on
dialysis in the USA: a cross-sectional study. Lancet. 2020;396:1335-44
. DOI: 10.1016/S0140-6736(20)32009-2. [PMID:32987007]
[62]
AA Butt and P Yan. Rates and characteristics of SARS-CoV-2 infection in persons with hepatitis C virus
infection. Liver Int. 2021;41:76-80
. DOI: 10.1111/liv.14681. [PMID:33006798]
[63]
LE Egede, RJ Walker, E Garacci, and JR Raymond. Racial/Ethnic Differences In COVID-19 Screening, Hospitallization, And Mortality In
Southeast Wisconsin. Health Aff (Millwood). 2020;39:1926-34. DOI: 10.1377/hlthaff.2020.01081. [PMID:33136498]
[64]
BL Horta, MF Silveira, AJD Barros, FC Barros, FP Hartwig, and MS Dias. Prevalence of antibodies against SARS-CoV-2 according to socioeconomic and ethnic
status in a nationwide Brazilian survey. Rev Panam Salud Publica. 2020;44:e135
. DOI: 10.26633/RPSP.2020.135. [PMID:33165337]
[65]
M Joy, FDR Hobbs, JL Bernal, J Sherlock, G Amirthalingam, and D McGagh. Excess mortality in the first COVID pandemic peak: cross-sectional analyses of the
impact of age, sex, ethnicity, household size, and long-term conditions in people of known
SARS-CoV-2 status in England. Br J Gen Pract. 2020;70:e890-8
. DOI: 10.3399/bjgp20X713393. [PMID:33077508]
[66]
K Inagaki, P Garg, and CV Hobbs. SARS-CoV-2 Positivity Rates Among Children of Racial and Ethnic Minority Groups in
Mississippi. Pediatrics. 2021;147:e2020024349
. DOI: 10.1542/peds.2020-024349. [PMID:33115793]
[67]
CM Kosar, EM White, RA Feifer, C Blackman, S Gravenstein, and OA Panagiotou. COVID-19 Mortality Rates Among Nursing Home Residents Declined From March To November
2020. Health Aff (Millwood). 2021;40:655-63. DOI: 10.1377/hlthaff.2020.02191. [PMID:33705204]
[68]
G Livingston, H Rostamipour, P Gallagher, C Kalafatis, A Shastri, and L Huzzey. Prevalence, management, and outcomes of SARS-CoV-2 infections in older people and
those with dementia in mental health wards in London, UK: a retrospective observational study.
Lancet Psychiatry. 2020;7:1054-
63. DOI: 10.1016/S2215-0366(20)30434-X. [PMID:33031760]
[69]
ES Rosenberg, JM Tesoriero, EM Rosenthal, R Chung, MA Barranco, and LM Styer. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York.
Ann Epidemiol. 2020;48:23-9.e4
. DOI: 10.1016/j.annepidem.2020.06.004. [PMID:32648546]
[70]
S Sami, LJ Akinbami, LR Petersen, A Crawley, SL Lukacs, and D Weiss. Prevalence of SARS-CoV-2 Antibodies in First Responders and Public Safety Personnel,
New York City, New York, USA, May-July 2020. Emerg Infect Dis. 2021;27:796-
804. DOI: 10.3201/eid2703.204340. [PMID:33493106]
[71]
ME Van Dyke, MCB Mendoza, W Li, EM Parker, B Belay, and EM Davis. Racial and Ethnic Disparities in COVID-19 Incidence by Age, Sex, and Period Among
Persons Aged <25 Years-16 US Jurisdictions, January 1-December 31, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:382-8. DOI: 10.15585/mmwr.mm7011e1. [PMID:33735165]
[72]
Ward H, Atchison C, Whitaker M, Ainslie KEC, Elliott J, Okell L,
et al. SARS-CoV-2 antibody prevalence in England following the first peak of the pandemic. Nat Comm.
2021;12.
[73]
CH Weng, A Saal, DC McGuire, and PA Chan. Persistently high SARS-CoV-2 positivity rate and incidence for Hispanic/Latinos
during state reopening in an urban setting: a retrospective cohort study. Epidemiol Infect. 2021;149:e25
. DOI: 10.1017/S0950268821000133. [PMID:33455608]
[74]
GL Werneck, LC Porto, A Sena, OD Ferreira, AC Cavalcanti, and AMG Santos. The incidence and geographical spread of SARS-CoV-2 in Rio de Janeiro, Brazil based
on RT-PCR test results. Rev Soc Bras Med Trop. 2021;54:
e07792020. DOI: 10.1590/0037-8682-0779-2020. [PMID:33605384]
[75]
BR Yehia, A Winegar, R Fogel, M Fakih, A Ottenbacher, and C Jesser. Association of Race With Mortality Among Patients Hospitalized With Coronavirus
Disease 2019 (COVID-19) at 92 US Hospitals. JAMA Netw Open. 2020;3:e2018039
. DOI: 10.1001/jamanetworkopen.2020.18039. [PMID:32809033]
[76]
ICNARC. ICNARC report on COVID-19 in critical care - 10 April
2020. London: Intensive Care National Audit and Research Centre (ICNARC); 2020.
[77]
EK Stokes, LD Zambrano, KN Anderson, EP Marder, KM Raz, and SEB Felix. Coronavirus disease 2019 case surveillance—United States, January
22–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759-65. DOI: 10.15585/mmwr.mm6924e2. [PMID:32555134]
[78]
S Agarwal, C Schechter, W Southern, JP Crandall, and Y Tomer. Preadmission Diabetes-Specific Risk Factors for Mortality in Hospitalized Patients
With Diabetes and Coronavirus Disease 2019. Diabetes Care. 2020;43:2339-44
. DOI: 10.2337/dc20-1543. [PMID:32769128]
[79]
A Alaa, ZZ Qian, J Rashbass, J Benger, and M van der Schaar. Retrospective cohort study of admission timing and mortality following COVID-19
infection in England. BMJ Open. 2020;10:e042712
. DOI: 10.1136/bmjopen-2020-042712. [PMID:33234660]
[80]
A Avula, K Nalleballe, S Toom, S Siddamreddy, D Gurala, and N Katyal. Incidence of Thrombotic Events and Outcomes in COVID-19 Patients Admitted to
Intensive Care Units. Cureus. 2020;12:e11079. [PMID:33224673]
[81]
VM Castro, TH McCoy, and RH Perlis. Laboratory Findings Associated With Severe Illness and Mortality Among Hospitalized
Individuals With Coronavirus Disease 2019 in Eastern Massachusetts. JAMA Netw Open. 2020;3:e2023934
. DOI: 10.1001/jamanetworkopen.2020.23934. [PMID:33125498]
[82]
SL Harrison, E Fazio-Eynullayeva, DA Lane, P Underhill, and GYH Lip. Higher Mortality of Ischaemic Stroke Patients Hospitalized with COVID-19 Compared to
Historical Controls. Cerebrovasc Dis. 2021;50:326-31
. DOI: 10.1159/000514137. [PMID:33774618]
[83]
AC Hernandez-Romieu, MW Adelman, MA Hockstein, CJ Robichaux, JA Edwards, and JC Fazio. Timing of Intubation and Mortality Among Critically Ill Coronavirus Disease 2019
Patients: A Single-Center Cohort Study. Crit Care Med. 2020;48:e1045-53
. DOI: 10.1097/CCM.0000000000004600. [PMID:32804790]
[84]
Z Imam, F Odish, I Gill, D O’Connor, J Armstrong, and A Vanood. Older age and comorbidity are independent mortality predictors in a large cohort of
1305 COVID-19 patients in Michigan, United States. J Intern Med. 2020;288:469-76
. DOI: 10.1111/joim.13119. [PMID:32498135]
[85]
L Kim, M Whitake, A O’Halloran, A Kambhampati, SJ Chai, and A Reingold. Hospitalization Rates and Characteristics of Children Aged <18 Years Hospitalized
with Laboratory-Confirmed COVID-19 - COVID-NET, 14 States, March 1-July 25, 2020.
MMWR Morb Mortal Wkly Rep. 2020;69:1081
-8. DOI: 10.15585/mmwr.mm6932e3. [PMID:32790664]
[86]
J Xie, Y Zu, A Alkhatib, TT Pham, F Gill, and A Jang. Metabolic Syndrome and COVID-19 Mortality Among Adult Black Patients in New Orleans.
Diabetes Care. 2020;44:188-93
. DOI: 10.2337/dc20-1714. [PMID:32843337]
[87]
Y Xie, B Bowe, G Maddukuri, and Z Al-Aly. Comparative evaluation of clinical manifestations and risk of death in patients
admitted to hospital with covid-19 and seasonal influenza: cohort study. BMJ. 2020;371:m4677 DOI: 10.1136/bmj.m4677. [PMID:33323357]
[88]
JAW Gold, KK Wong, CM Szablewski, PR Patel, J Rossow, and J da Silva. Characteristics and Clinical Outcomes of Adult Patients Hospitalized with COVID-19 -
Georgia, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:545-50. DOI: 10.15585/mmwr.mm6918e1. [PMID:32379729]
[89]
MP Abrams, EY Wan, MP Waase, JP Morrow, JM Dizon, and H Yarmohammadi. Clinical and cardiac characteristics of COVID-19 mortalities in a diverse New York
City Cohort. J Cardiovasc Electrophysiol. 2020;31:3086
-96. DOI: 10.1111/jce.14772. [PMID:33022765]
[90]
D Ayoubkhani, V Nafilyan, C White, P Goldblatt, C Gaughan, and L Blackwell. Ethnic-minority groups in England and Wales-factors associated with the size and
timing of elevated COVID-19 mortality: a retrospective cohort study linking census and death
records. Int J Epidemiol. 2021;49:1951-
62. DOI: 10.1093/ije/dyaa208. [PMID:33349855]
[91]
MT Bassett, JT Chen, and N Krieger. Variation in racial/ethnic disparities in COVID-19 mortality by age in the United
States: A cross-sectional study. PLoS Med. 2020;17:e1003402
. DOI: 10.1371/journal.pmed.1003402. [PMID:33539382]
[92]
D Bixler, AD Miller, CP Mattison, B Taylor, K Komatsu, and XP Pompa. SARS-CoV-2-Associated Deaths Among Persons Aged <21 Years - United States,
February 12-July 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1324
-9. DOI: 10.15585/mmwr.mm6937e4. [PMID:32941417]
[93]
M Scannell Bryan, JH Sun, J Jagai, DE Horton, A Montgomery, and R Sargis. Coronavirus disease 2019 (COVID-19) mortality and neighborhood characteristics in
Chicago. Ann Epidemiol. 2021;56:47 DOI: 10.1016/j.annepidem.2020.10.011. [PMID:33181262]
[94]
TL Cowger, BA Davis, OS Etkins, K Makofane, JA Lawrence, and MT Bassett. Comparison of Weighted and Unweighted Population Data to Assess Inequities in
Coronavirus Disease 2019 Deaths by Race/Ethnicity Reported by the US Centers for Disease Control
and Prevention. JAMA Netw Open. 2020;3:e2016933
. DOI: 10.1001/jamanetworkopen.2020.16933. [PMID:32721026]
[95]
S de Lusignan, M Joy, J Oke, D McGagh, B Nicholson, and J Sheppard. Disparities in the excess risk of mortality in the first wave of COVID-19: Cross
sectional study of the English sentinel network. J Infect. 2020;81:785-92
. DOI: 10.1016/j.jinf.2020.08.037. [PMID:32858068]
[96]
J Elliott, B Bodinier, M Whitaker, C Delpierre, R Vermeulen, and I Tzoulaki. COVID-19 mortality in the UK Biobank cohort: revisiting and evaluating risk factors.
Eur J Epidemiol. 2021;36:299-
309. DOI: 10.1007/s10654-021-00722-y. [PMID:33587202]
[97]
CT Laurencin, ZH Wu, A McClinton, JJ Grady, and JM Walker. Excess Deaths Among Blacks and Latinx Compared to Whites During Covid-19.
J Racial Ethn Health Disparities. 2021;8:783
-9. DOI: 10.1007/s40615-021-01010-x. [PMID:33751484]
[98]
T Rushovich, M Boulicault, JRT Chen, AC Danielsen, A Tarrant, and SS Richardson. Sex Disparities in COVID-19 Mortality Vary Across US Racial Groups. J Gen Intern Med. 2021;36:1696-
701. DOI: 10.1007/s11606-021-06699-4. [PMID:33818679]
[99]
K Credit. Neighbourhood inequity: Exploring the factors underlying racial and ethnic
disparities in COVID-19 testing and infection rates using ZIP code data in Chicago and New York.
Regional Science Policy and Practice. 2020;12:
1249-71. DOI: 10.1111/rsp3.12321
[100]
CA Scannell, CIA Oronce, and Y Tsugawa. Association Between County-Level Racial and Ethnic Characteristics and COVID-19 Cases
and Deaths in the USA. J Gen Intern Med. 2020;35:3126-
8. DOI: 10.1007/s11606-020-06083-8. [PMID:32761284]
[102]
TN Rogers, CR Rogers, E VanSant-Webb, LY Gu, B Yan, and F Qeadan. Racial Disparities in COVID-19 Mortality Among Essential Workers in the United
States. World Med Health Policy. 2020;12:311-27. DOI: 10.1002/wmh3.358. [PMID:32837779]
[103]
K Arasteh. Prevalence of Comorbidities and Risks Associated with COVID-19 Among Black and
Hispanic Populations in New York City: an Examination of the 2018 New York City Community Health
Survey. J Racial Ethn Health Disparities. 2020;Online ahead of print
DOI: 10.1007/s40615-020-00844-1. [PMID:32794024]
[104]
ZD Bailey, N Krieger, M Agénor, J Graves, N Linos, and MT Bassett. Structural racism and health inequities in the USA: evidence and interventions.
Lancet. 2017;389:1453-63
. DOI: 10.1016/S0140-6736(17)30569-X. [PMID:28402827]
[105]
N Krieger. Discrimination and Health Inequities. Int J Health Serv. 2014;44:643-
710. DOI: 10.2190/HS.44.4.b. [PMID:25626224]
[106]
Y Paradies, B Jehonathan, N Denson, E Amanuel, N Priest, and A Pieterse. Racism as a Determinant of Health: A Systematic Review and Meta-Analysis.
PLoS One. 2015;10:e0138511
. DOI: 10.1371/journal.pone.0138511. [PMID:26398658]
[107]
NN Pettit, EL MacKenzie, JP Ridgway, K Pursell, D Ash, and B Patel. Obesity is Associated with Increased Risk for Mortality Among Hospitalized Patients
with COVID-19. Obesity (Silver Spring). 2020;28:1806-10. DOI: 10.1002/oby.22941. [PMID:32589784]
[108]
D McGonagle, S Plein, JS O’Donnell, K Sharif, and C Bridgewood. Increased cardiovascular mortality in African Americans with COVID-19.
Lancet Respir Med. 2020;8:649-
51. DOI: 10.1016/S2213-2600(20)30244-7. [PMID:32473125]
[109]
Y Nguyen, F Corre, V Honsel, S Curac, V Zarrouk, and CP Burtz. A nomogram to predict the risk of unfavourable outcome in COVID-19: a retrospective
cohort of 279 hospitalized patients in Paris area. Ann Med. 2020;52:367-75. DOI: 10.1080/07853890.2020.1803499. [PMID:32723107]
[110]
J Lian, X Jin, S Hao, H Cai, S Zhang, and L Zheng. Analysis of Epidemiological and Clinical Features in Older Patients With Coronavirus
Disease 2019 (COVID-19) Outside Wuhan. Clin Infect Dis. 2020;71:740-7
. DOI: 10.1093/cid/ciaa242. [PMID:32211844]
[111]
. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19)-United
States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343-6. DOI: 10.15585/mmwr.mm6912e2. [PMID:32214079]
[112]
OL Strickland, Y Powell Young, C Reyes-Miranda, O Alzaghari, C Brannon, and J Newman Giger. African-Americans Have a Higher Propensity for Death from COVID-19: Rationale and
Causation. J Natl Black Nurses Assoc. 2020;31:1-12. [PMID:32853490]
[113]
M El Chaar, K King, and AG Lima. Are black and Hispanic persons disproportionately affected by COVID-19 because of
higher obesity rates? Surg Obes Relat Dis. 2020;16:1096-9. DOI: 10.1016/j.soard.2020.04.038. [PMID:32522406]
[116]
KC Ferdinand and SA Nasser. African-American COVID-19 Mortality A Sentinel Event. J Am Coll Cardiol. 2020;75:2746-8. DOI: 10.1016/j.jacc.2020.04.040. [PMID:32330545]
[117]
E Abuelgasim, LJ Saw, M Shirke, M Zeinah, and A Harky. COVID-19: Unique public health issues facing Black, Asian and minority ethnic
communities. Curr Probl Cardiol. 2020;45:100621
. DOI: 10.1016/j.cpcardiol.2020.100621. [PMID:32448759]
[118]
JF Sallis and K Glanz. The Role of Built Environments in Physical Activity, Eating, and Obesity in
Childhood. Future Child. 2006;16:89-108
. DOI: 10.1353/foc.2006.0009. [PMID:16532660]
[119]
A Walters. Inequities in access to education: Lessons from the COVID-19 pandemic.
Brown Univ Child Adolesc Behav Lett. 2020;36:8
DOI: 10.1002/cbl.30483
[120]
S Yaya, H Yeboah, CH Charles, A Otu, and R Labonte. Ethnic and racial disparities in COVID-19-related deaths: counting the trees, hiding
the forest. BMJ Glob Health. 2020;5:e002913
. DOI: 10.1136/bmjgh-2020-002913. [PMID:32513864]
|
|
 |





















