|
|
|
Omar Irfan, Jiang Li, Kun Tang, Zhicheng Wang, and Zulfiqar A Bhutta
|
Abstract
Background
There is uncertainty with respect to SARS-CoV-2 transmission in children (0-19 years) with
controversy on effectiveness of school-closures in controlling the pandemic. It is of equal
importance to evaluate the risk of transmission in children who are often asymptomatic or mildly
symptomatic carriers that may incidentally transmit SARS-CoV-2 in different settings. We
conducted this review to assess transmission and risks for SARS-CoV-2 in children (by age-groups
or grades) in community and educational-settings compared to adults.
Methods
Data for the review were retrieved from PubMed, EMBASE, Cochrane Library, WHO COVID-19 Database,
China National Knowledge Infrastructure (CNKI) Database, WanFang Database, Latin American and
Caribbean Health Sciences Literature (LILACS), Google Scholar, and preprints from medRixv and
bioRixv) covering a timeline from December 1, 2019 to April 1, 2021. Population-screening,
contact-tracing and cohort studies reporting prevalence and transmission of SARS-CoV-2 in
children were included. Data were extracted according to PRISMA guidelines. Meta-analyses were
performed using Review Manager 5.3.
Results
Ninety studies were included. Compared to adults, children showed comparable national (risk ratio
(RR) = 0.87, 95% confidence interval (CI) = 0.71-1.060 and
subnational (RR = 0.81, 95% CI = 0.66-1.01) prevalence in
population-screening studies, and lower odds of infection in community/household contact-tracing
studies (odds ratio (OR) = 0.62, 95% CI = 0.46-0.84). On
disaggregation, adolescents observed comparable risk (OR = 1.22, 95%
CI = 0.74-2.04) with adults. In educational-settings, children attending
daycare/preschools (OR = 0.53, 95% CI = 0.38-0.72) were observed to
be at lower-risk when compared to adults, with odds of infection among primary
(OR = 0.85, 95% CI = 0.55-1.31) and high-schoolers
(OR = 1.30, 95% CI = 0.71-2.38) comparable to adults. Overall,
children and adolescents had lower odds of infection in educational-settings compared to
community and household clusters.
Conclusions
Children (<10 years) showed lower susceptibility to COVID-19 compared to adults, whereas
adolescents in communities and high-schoolers had comparable risk. Risks of infection among
children in educational-settings was lower than in communities. Evidence from school-based
studies demonstrate it is largely safe for children (<10 years) to be at schools, however
older children (10-19 years) might facilitate transmission. Despite this evidence, studies
focusing on the effectiveness of mitigation measures in educational settings are urgently needed
to support both public health and educational policy-making for school reopening.
|
As of 5 April 2021, there have been 131.0 million confirmed COVID-19 cases and nearly 2.8 million confirmed
deaths globally [1]. The response in countries worldwide has gone from
an initial stage of strict lockdowns and business closures to variable periods of relaxation with social
distancing, use of face masks and hand hygiene, and now vaccination roll outs for adults. During this
period, daycare centers, schools and educational institutions were closed initially and then reopened at
different stages. This has however, been a bone of much contention across the world with various countries
adopting different measures. The global spread of variants of concern now threatens to reverse progress and
disrupt the opening of the economy, commerce and education.
School closures are understandable. Children play an important role in the transmission of some respiratory
infectious diseases and may suffer from more severe outcomes than adults, such as influenza [2,3], rendering school closures an
effective public health policy in reducing the spread and influence of these diseases. This is especially
true in novel pandemics where pharmaceutical interventions, such as vaccines, are not immediately available
and delaying disease spread is a priority [3-6]. However, children and adolescents under 19 years of age comprise a small proportion
of total reported COVID-19 cases (1%-10%) [7-9]. This group has been reported to present with a milder clinical course compared to
adults infected with SARS-CoV-2, with more favorable outcomes in general [7
,9-11].
To date, there is much controversy concerning the benefits of the ongoing and future closure of schools and
other educational institutions in controlling the COVID-19 pandemic, as limited data on transmission of
COVID-19 in educational settings is available [12-16]. It is of equal importance to evaluate the risk of susceptibility
and transmission in children who are often asymptomatic or mildly symptomatic carriers, that may
incidentally transmit SARS-CoV-2 in both educational and community settings, especially with the third wave
of COVID-19 and newer variants spreading in many Countries crippling the health care system and economy.
We undertook a systematic review of the infection and transmission rates and risks of SARS-CoV-2 in children
and adolescents in household, community and educational settings since the beginning of the pandemic, to
help in understanding policy responses for safe school reopening for children of various ages.
METHODS
This systematic review is reported in accordance with the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses (PRISMA) reporting guidelines.
In this review, we focused on the following review objectives:
1-What is the overall risk of infection in children and adolescents compared to adults (>19 years)
from population screening and contact-tracing studies?
2-What are the odds of being an infected contact in children and adolescents compared to adults (>19
years) in educational settings?
3-What is the risk of infection for children and adolescents in educational settings in comparison to
that in communities?
Literature search
To investigate the risk of SARS-CoV-2 infection and transmission in children and adolescents and
their potential contribution to transmission in various settings, we searched for national and
subnational prevalence studies, and contact-tracing studies (CTS) from community/household clusters
and educational settings.
Data for the review were retrieved from PubMed, EMBASE, Cochrane Library, WHO COVID-19 Database,
China National Knowledge Infrastructure (CNKI) Database, WanFang Database, Latin American and
Caribbean Health Sciences Literature (LILACS), Google Scholar, and “Living Evidence of
COVID-19” (a database updated daily with published articles from PubMed and EMBASE and
preprints from medRixv and bioRixv) covering a timeline from December 1, 2019 to April 1, 2021.
Preprints from ChinaXiv (http://www.chinaxiv.org/home.htm) were
also searched. Complementary searches were conducted by manually searching the national public
health websites, and the John Hopkins Humanitarian Health Resource. The reference lists of all
retrieved articles were examined as well. There was no language restriction applied for the search.
The search terms applied for each research question and the specific search strategies for PubMed
and other databases are provided in Table S1 in the
Online Supplementary Document
.
The search results from various databases were uploaded into Covidence Systematic Review Software
(Veritas Health Innovation 2016, Melbourne, Australia) for screening.
Inclusion and exclusion criteria
We included population screening studies investigating the age-specific prevalence of SARS-CoV-2
infections, contact-tracing and cohort studies reporting the incidence and attack rate (number of
infections secondary to a suspected close contact) of children (0-9 years) and adolescents (10-19
years old) compared to adults, case series presenting direct evidence of COVID-19 cases transmitted
by SARS-CoV-2 positive children compared to adults, and data from national public health websites
and official government reports, when available. We excluded review articles, opinions, viewpoints
and communication letters (if not presenting data on number of infections or attack rate of
SARS-CoV-2) and modeling studies were also excluded. Studies that did not report the number of
infections or attack rate of SARS-CoV-2, and studies with possible duplications of cases (eg,
overlapping time periods within the same institutions/cities/countries) were also excluded.
Study screening
Two review authors independently reviewed each title and abstract from the search results. Upon
obtaining the full text, two reviewers independently screened the full text and decided whether to
include or exclude the study, in accordance with the criteria specified previously. Any
disagreements were resolved by independent review by a third author.
Data extraction
The following data were extracted from each study using standardized data abstraction forms: authors,
country, study type, study period and its relationship with the epidemic curve in the country/area
and school closure/reopen status, study setting (household, community, daycare, primary or secondary
school; other mitigation measures if any), case definition (index case, primary case, secondary
case), testing methods, contact-tracing methods, sampling method, number of infected children and/or
adults (specified whether or not student-contacting staff) and total number of students and staff in
the educational setting (or reported attack rate).
Meta-analysis and qualitative synthesis
For each dichotomous outcome, the weighted mean prevalence and 95% confidence interval (CI) was
calculated. The meta-analyses were performed using Review Manager 5.3 adopting the random-effects
models. Pooled risk ratios (RR) between children and adults were presented in both national and
subnational prevalence studies with disaggregation into active infection and past infection
indicated by PCR testing and antibodies seroprevalence, respectively. The pooled odds ratios (OR) of
children being infected in households were presented and disaggregated by children (<10 years)
and adolescent (10-19 years), and school operational status (open/partially open or closed) in the
region/Country. The odds of contracting infection in children compared to adults in schools and
daycare centers were also analyzed. Total number of children and adolescents tested and diagnosed
with COVID-19 were computed separately for communities and educational settings to calculate the
odds ratio (OR) of risk of infection in educational settings compared to community settings.
Statistical heterogeneity across studies was evaluated by calculating the I2 statistic.
I2 values equal to or above 50% were considered as “significant”
heterogeneity in this study. Additionally, the χ2 test for heterogeneity was
performed and the forest plot was visually inspected to assess the degree of overlap between the CIs
of included studies. The characteristics, biases, and results of the included studies were
summarized narratively. For studies included but not eligible for meta-analysis due to a lack of
sufficient data, we also qualitatively synthesized the results to present a full picture.
Assessment of methodological quality and risk of bias
Two independent reviewers assessed each included study for methodological quality. A quality
assessment tool was adopted from the National Heart, Lung, and Brain Institute (NHLBI) and Research
Triangle Institute International [17] for observational
studies, where are as the quality of prevalence studies was assessed using a critical appraisal
checklist for prevalence studies [18]. Study quality was
scored on basis of clear study objectives, case definition, consecutive inclusion of cases, sample
sizes, comparability of included patients, measurement of outcomes, length of follow-up, and
appropriately defined statistical methods and results. However, studies were not excluded based on
study quality. Studies with score 6-8 were considered to be good quality, 4-5 considered fair
quality and <4 considered poor quality.
RESULTS
The systemic literature search yielded 3700 results during the search dates. Of these, 117 studies were
examined in full text and 90 were included in the final analysis after addition of 3 studies from other
resources (
Figure 1
). The characteristics of included studies were summarized in Table S2 in the
Online Supplementary Document
. Thirty studies were excluded because they either presented overlapping data, provided little
age-disaggregated data for children, or were commentaries, editorials or reviews with no empirical data.
Figure 1.
PRISMA flow diagram of study selection process. 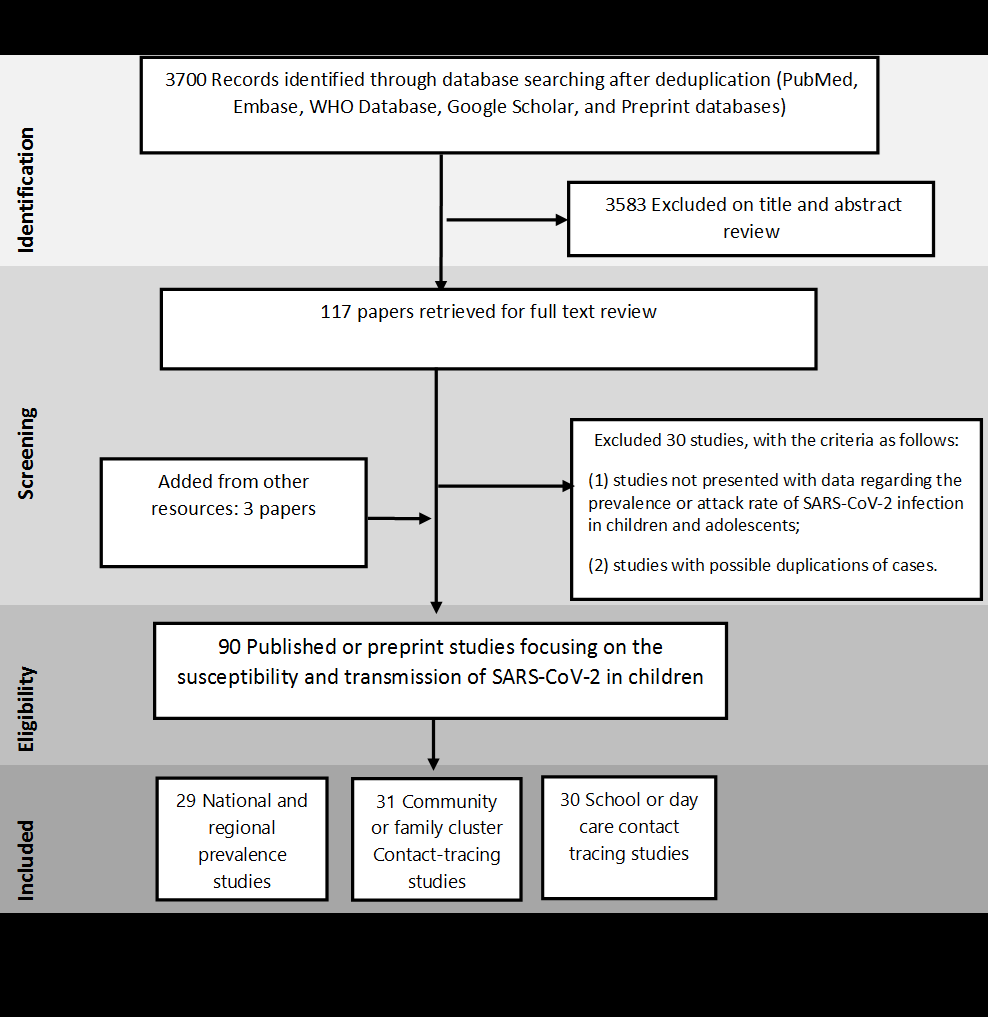 The overall risk of SARS-Cov-2 infection among children and adolescents in comparison to adults
To investigate the overall risk of SARS-Cov-2 infection among children and adolescents, we included
60 studies, of which 29 were population-screening studies [19
-47] and 31 were CTS [48
-78].
The prevalence of COVID-19 in children and adolescents (<20 years of age) were reported in 11
national and 18 subnational surveillance studies. Among them, six were from low- and middle-income
countries (LMICs). Compared to adult populations, a comparable risk of SARS-CoV-2 infection was
observed in children and adolescents in both national (RR = 0.87, 95%
CI = 0.71-1.06) and subnational (RR = 0.81, 95%
CI = 0.66-1.01) surveillance studies, as shown in
Figure 2
and
Figure 3
. When disaggregated by testing methods (ie, RT-PCR vs serological test), children and
adolescents showed a similar lower risk of past infection from seroprevalence data in national
(RR = 0.77, 95% CI = 0.62-0.96) studies but insignificant effect in
subnational studies (RR = 0.80, 95% CI = 0.59-1.08). The risk of active
infection was lower compared to adults but insignificant in both national studies
(RR = 0.98, 95% CI = 0.69-1.38) and subnational surveillance studies at
point estimate level (RR = 0.77, 95% CI = 0.48-1.22).
Figure 2.
Pooled risk ratio of SARS-Cov-2 infection in children vs adults in national surveillance,
disaggregated by infection positivity and seroprevalence. 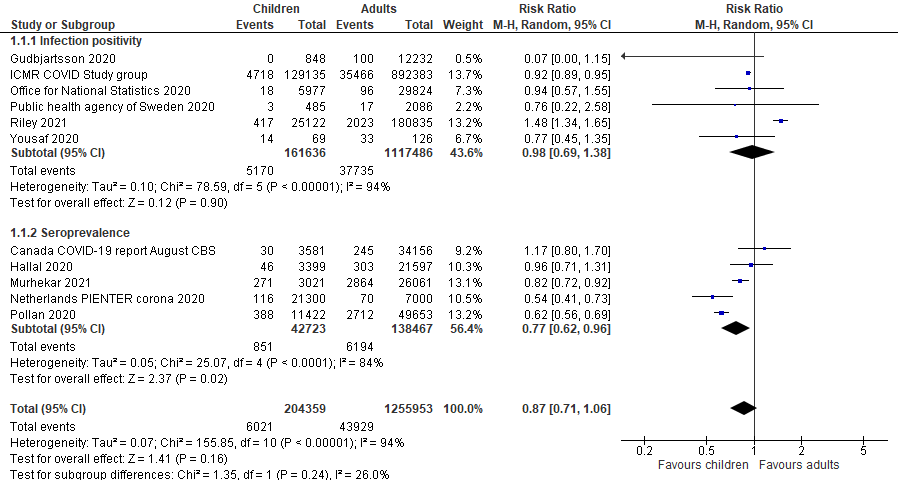 Figure 3.
Pooled risk ratio of SARS-Cov-2 infection in children vs adults in subnational surveillance,
sub-grouped into infection positivity and seroprevalence. 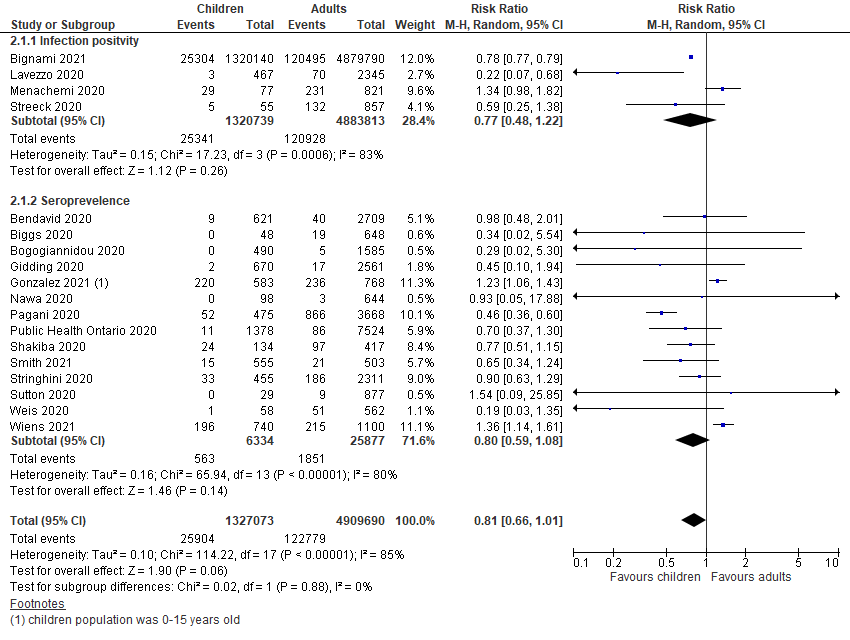 Thirty-one studies undertook contact-tracing in community, household and family clusters, of which,
12 were from LMICs. The pooled odds of secondary attack in children and adolescents was
significantly lower than that in adults (OR = 0.62, 95% CI = 0.46-0.84),
with high heterogeneity (I2 = 0.91) (
Figure 4
, Panel A). When further disaggregated by the schools’ operational status (ie, school
closure vs school fully or partially open) during the study period, both children and adolescents
were found to have lower risk of infection than did adults when schools were fully or partially open
(OR = 0.52, 95% CI = 0.33-0.83), but no significant effect during school
closures (OR = 0.72, 95% CI = 0.46-1.14).
Figure 4.
Pooled odds of children and adolescents being an infected contact in community and household
family clusters Panel A. Odds of children and adolescents being infected vs
adults, by school status. Panel B. Odds of children and adolescents being
infected vs adults, by age group (subset of studies in Panel A). 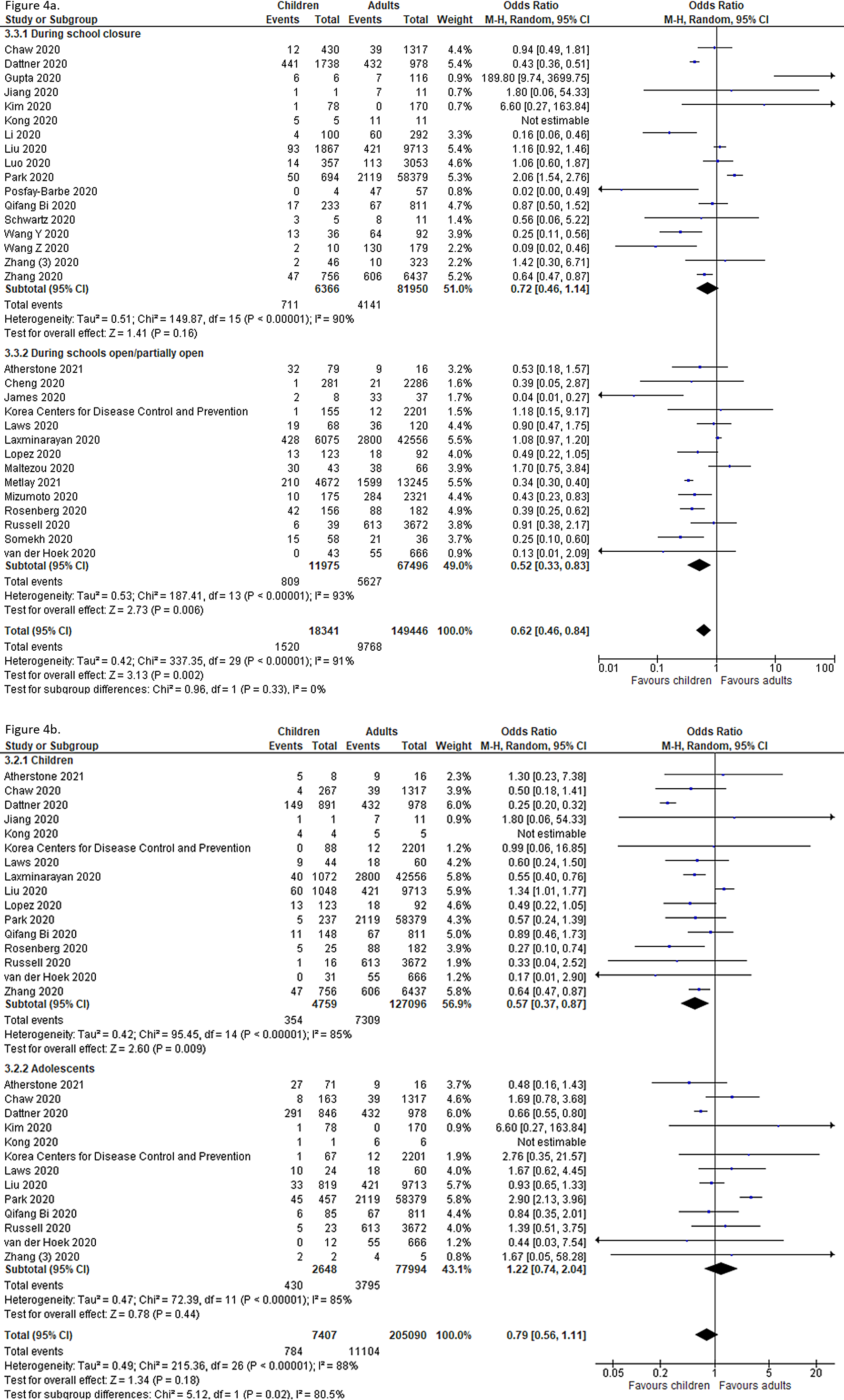 In a subgroup analysis of CTS (based on 18 out of the 31 studies) in which age-disaggregation was
possible, we found that the pooled OR for SARS-CoV-2 infection among children compared to adults was
0.57 (95% CI = 0.37-0.87), suggesting a significantly lower risk of secondary attack
in this population at the community and household level. However, a comparable risk of secondary
attack was observed among adolescents (OR = 1.22, 95% CI = 0.74-2.04) (
Figure 4
, Panel B).
Infection and transmission of COVID-19 among children and adolescents in educational settings
compared to adult teachers and staff
Thirty studies conducted in educational settings were included [12
-15,57,79-103], among which
six studies were cross-sectional studies and the remaining 24 were contact-tracing or cohort
studies. Upon checking the availability of sufficient data for comparison of children vs adults, 24
studies were included in the meta-analysis.
The pooled estimate of the included studies suggested that children and adolescents appeared to have
a lower though statistically insignificant risk of secondary attack in school settings when compared
to adults (OR = 0.84, 95% CI = 0.62-1.14) (
Figure 5
). Subgroup analysis also suggested significant lower odds of infection among children
attending daycare centers/preschools (OR = 0.53, 95% CI = 0.38-0.72),
but insignificant effect in primary schools (OR = 0.85, 95%
CI = 0.55-1.31) compared to the adult staff. However, high-school students had
comparable risk of infection to adults (OR = 1.30, 95% CI = 0.71-2.38).
Figure 5.
Pooled odds ratios for children and adolescent contracting infection compared to adults, by
educational setting. 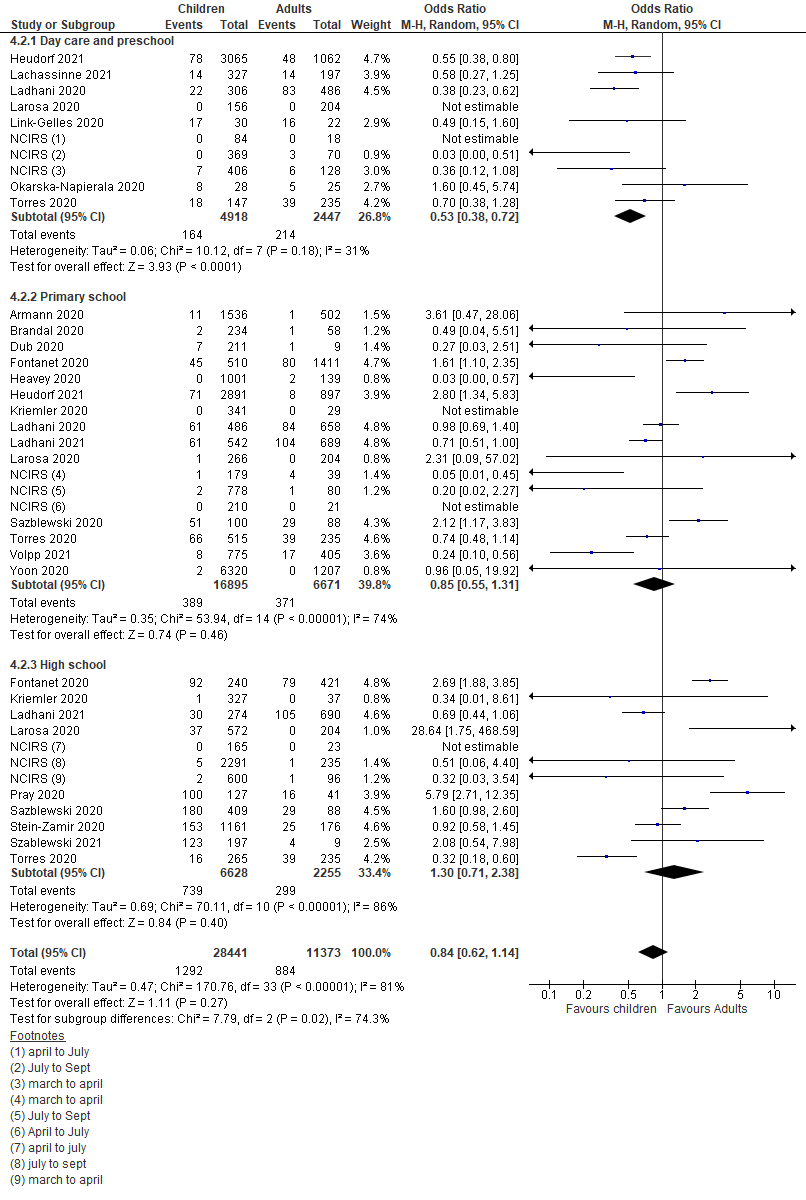 Risk of contracting SARS-CoV-2 infection among children and adolescents in schools compared to
community settings
Using the existing evidence from both community-based studies and studies conducted in educational
settings, we further calculated the pooled odds ratios for contracting infection among children and
adolescent in educational settings vs communities and household-clusters. When total number of
children and adolescents tested and diagnosed with COVID-19 in the two settings were compared,
children observed lower odds of infection (OR = 0.53, 95%
CI = 0.38-0.75) in schools compared to community and households, which was
consistently observed on disaggregation by age; children (<10 years) (OR = 0.45,
95% = 0.39-0.51); adolescents and high-schoolers (OR = 0.63, 95%
CI = 0.56-0.72) (Figure S1 in the
Online Supplementary Document
).
Study quality assessment
The majority of included studies were considered of good or fair quality based on the scores
generated by using quality assessment tools (Table S3 in the
Online Supplementary Document
). Out of the 29 population prevalence studies, 28 were of good quality while one was of fair
quality. Twenty-five out of 31 contact-tracing studies were of good quality while six were of fair
quality. For studies conducted in educational settings, eight were of fair quality and the remaining
22 were of good quality.
Studies were primarily downgraded for inadequate sample size and unclear description of study
setting. However, potential biases were noted for some of the included studies, which could
negatively affect their quality (eg, low response rate from the study population in contact-tracing
studies [15,81,83,85], only symptomatic
cases receiving tests [12,14
,79,86,104]).
DISCUSSION
This systematic review provides a comprehensive assessment of COVID-19 risk of infection and transmission
in children and adolescents compared to adults in household, community and educational settings and in
the relationship of age and school contexts with risks of transmission. Consistent with previous reviews
[105,106], we found an
overall lower risk of infection among children and adolescents (0-19 years) in households and
communities compared to adults. In educational settings, children attending daycare, preschool and
primary school presented a lower risk of infection than that of adults.
Our review has several important strengths. Compared to existing reviews [105,106], most of which were conducted at
an earlier stage of the pandemic, the present review provided the most up-to-date evidence of this
research question. We have undertaken several pre-specified sub-group analyses as per data availability.
The subgroup comparisons included assessment of active infection (PCR test), past infection (blood
serology), school operational status and differential effects by age groups. It was also important to
assess if the risk of community transmission was affected by school closure. Using a broad search
strategy implemented in English, Chinese and Spanish databases, we summarize evidence from 90 studies
from 31 different countries. We also attempted to reduce possible overlap in cases to prevent
duplication. Compared to a previous systematic review by Viner et al [
105], we report almost thrice the number of studies with disaggregation of analyses by age
and settings. This review is primarily limited by the large heterogeneity across studies and the lack of
uniform age- and test-specific evidence for transmission in different study settings. Lastly, the
evidence of COVID-19 infection in children is rapidly evolving; therefore, evidence from this review
should be cautiously interpreted and regularly updated. This review does not include modelling studies,
which can forecast future transmission scenarios but under various assumptions about disease
transmission and immunity [107].
Currently available epidemiological data have revealed two unique features of pediatric COVID-19 cases: a
relatively low prevalence in this population and milder clinical features compared to adult patients [
9,108]. Several studies
and reviews have studied children and adolescents’ susceptibility to SARS-CoV-2 infection and
their role in transmission in different settings. Viner et al. examined studies on the prevalence of
SARS-CoV-2 infection in children and young people (<20 years), and found that the pooled odds ratio
of being infected among children vs adults was 0.56 (95% CI: 0.37-0.85) with substantial heterogeneity
(I2 = 95%) [105]. Goldstein et al [
109] reviewed data on detection of SARS-CoV-2 infection in
different settings and suggested a significantly lower susceptibility of infection for children (<10
years of age) compared to adults. There was some evidence of robust spread of SARS-CoV-2 in secondary
and high-schools (eg, high seroprevalence of anti-SARS-CoV-2 antibodies among high-school students in
northern France [83], and an outbreak in an Israel high-school [
13]), while the spread seemed to be more limited in primary
schools [12,14,15,82,88-91]. Xu et al. conducted a living
systematic review and reported that the SARS-CoV-2 infection attack rates were 0.15% (95%
CI = 0%-0.93%) among students and 0.70% (95% CI = 0%-3.56%) among school
staff, respectively [110]. These findings are largely consistent
with the primary finding of the present study that children are not as susceptible to SARS-CoV-2
infection as adults, and while children are known to be “super spreaders” for influenza [
111] and measles viruses [112
], they play only a limited role in SARS-CoV-2 transmission in various settings.
Symptomatic patients have a lower SARS-CoV-2 cycle threshold (Ct) values, which corresponds to higher
viral RNA levels. SARS-CoV-2 Ct values have been found to be almost linearly inversely correlated with
its transmission [113]. Furthermore, a meta-analysis reported
risk of asymptomatic transmission is significantly lower than that of symptomatic transmission (relative
risk = 0.58; 95% CI = 0.34-0.99) [114
]. To contextualize, these findings might suggest that children may be less likely to transmit
SARS-CoV-2 due to their lower prevalence of symptomatic and severe presentation during the infection [
115].
School closures are an effective public health mitigation measure in reducing the community transmission
of many respiratory infectious diseases, such as influenza [2,
3], however, current evidence on the effectiveness of school
closures in curbing the COVID-19 pandemic is inconsistent. Large experiences from Australia, USA and
England demonstrated low transmission rates in schools and early childhood education services when these
facilities were still open [90,91
,116]. However, Auger et al. conducted a US
population-based observational study between March 9 and May 7, 2020 and found that school closure was
associated with a significant decline in the incidence of COVID-19 [117
]. Majority of the school linked index cases report none or only a small number of secondary
cases [91,93]. Reports
investigating outbreaks have demonstrated a higher transmission by school-age children to other students
or teachers, particularly when the mitigation measures were inadequately implemented in schools [104]. Reports from Sweden [118
] and US [119] suggest a comparable increased risk of
transmission from teacher to students and other staff members. These highlight the significance of
focusing COVID-19 prevention protocols and vaccination strategies for the teachers, which may indirectly
protect students who might not be immediately prioritized in the vaccine rollout. In the present study,
we find overall that opening educational establishments may not predispose children and adolescents to a
higher risk of SARS-CoV-2 infection compared to adults. On the contrary, children and adolescents were
found to have more than 2-fold greater risk of infection in household and community settings than in
schools. The school attendance may serve as a protective factor, which reduces children’s chances
of community contacts in a relatively isolated environment during school hours. It may also be
attributable to the effective infections control measures applied in schools introduced by global and
national guidelines.
Importantly, prolonged school closures have also been found to have negative impacts on the educational
and social development of children including increasing mental disorders, worsening nutrition, lack of
physical activities, substance abuse, child violence and abuse [106
,120-124].
Lessons from the 2013-2016 Ebola pandemic suggested that youth, and young girls in particular, of poor
households saw the largest increase in permanent school dropouts post-Ebola [125,126]. The disruption of education is
particularly harmful to young children who are in the most sensitive window of learning, as the early
education loss could permanently affect the development of one’s foundational skills [127]. Alternative educational opportunities such as online
distance learning may not be available to poorer or marginalized populations and are non-existent in
many LMICs [128].
The decision to reopen schools is understandably a delicate balance between various factors, including
the incidence of COVID-19 cases in the community, the concerns and choices of the parents and the
public, the school-based mitigation strategies in place including vaccinations for teachers and the
availability of resources. It is recommended that schools should only be reopened when the prevalence of
COVID-19 at the community level is under a relatively safe threshold [
129].
Safe reopening of schools is not possible without proper mitigation plans and strategies in place. Some
of the measures, which are suggested by the present guidelines, include repeat testing, avoiding
crowded/close contact environments, social distancing, wearing facial coverings, maintaining hand
hygiene, and some protective measure of classrooms and environment, including limiting classroom size
and ensuring adequate ventilation including open air classes where feasible [130]. Despite these recommended actions, there are major challenges in evaluating
the effectiveness of such guidelines. It is even more challenging to ensure the most effective
interventions to be properly implemented in schools. Mitigation strategies at schools may incur a
considerable financial cost. For instance, it was estimated that an additional 20 billion USD would be
needed for the nationwide implementation of recommended school-based mitigation strategies in the US [
131].There is currently limited data on and much need for
collating evidence from safe school reopening strategies and experience across the world.
Given the highly contagious nature of SARS-CoV-2 and the new variants, a expanding vaccine eligibility
for children and adolescents and addressing it’s hesitancy is the most effective strategy for
returning children to schools [116]. While some countries have
prioritized vaccination of school teachers and staff to reduce occupational transmission, the evidence
of effectiveness of vaccination strategies in adolescents is just emerging [132] and trials are being ramped up in younger children [133]. Given the potential serious complications of COVID-19 infection in subsets
of children [134], vaccination research and implementation in
children must be prioritized across the world.
REFERENCES
[1]
World Health Organization. WHO Coronavirus Disease (COVID-19)
Dashboard Data last updated: 2021.
https://covid19.who.int/. Accessed: 5 April 2021.
[2]
RM Viner, SJ Russell, H Croker, J Packer, J Ward, and C Stansfield. School closure and management practices during coronavirus outbreaks including
COVID-19: a rapid systematic review. Lancet Child Adolesc Health. 2020;4:397
-404. DOI: 10.1016/S2352-4642(20)30095-X. [PMID:32272089]
[3]
S Cauchemez, MD Van Kerkhove, BN Archer, M Cetron, BJ Cowling, and P Grove. School closures during the 2009 influenza pandemic: national and local experiences.
BMC Infect Dis. 2014;14:207 DOI: 10.1186/1471-2334-14-207. [PMID:24739814]
[4]
S Cauchemez, A-J Valleron, P-Y Boelle, A Flahault, and NM Ferguson. Estimating the impact of school closure on influenza transmission from Sentinel data.
Nature. 2008;452:750-4. DOI: 10.1038/nature06732. [PMID:18401408]
[5]
M Litvinova, Q-H Liu, ES Kulikov, and M Ajelli. Reactive school closure weakens the network of social interactions and reduces the
spread of influenza. Proc Natl Acad Sci U S A. 2019;116:13174
-81. DOI: 10.1073/pnas.1821298116. [PMID:31209042]
[6]
V Gemmetto, A Barrat, and C Cattuto. Mitigation of infectious disease at school: targeted class closure vs school closure.
BMC Infect Dis. 2014;14:695 DOI: 10.1186/s12879-014-0695-9. [PMID:25595123]
[7]
Y Dong, X Mo, Y Hu, X Qi, F Jiang, and Z Jiang. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702
. DOI: 10.1542/peds.2020-0702. [PMID:32179660]
[8]
. Coronavirus disease 2019 in children—United States, February 12–April
2, 2020. MMWR Morb Mortal Wkly Rep. 2020;[PMID:32271728]
[9]
O Irfan, F Muttalib, K Tang, L Jiang, ZS Lassi, and Z Bhutta. Clinical characteristics, treatment and outcomes of paediatric COVID-19: a systematic
review and meta-analysis. Arch Dis Child. 2021;106:440-8
. DOI: 10.1136/archdischild-2020-321385. [PMID:33593743]
[10]
F Götzinger, B Santiago-García, A Noguera-Julián, M Lanaspa, L Lancella, and FIC Carducci. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort
study. Lancet Child Adolesc Health. 2020;4:653
-61. DOI: 10.1016/S2352-4642(20)30177-2. [PMID:32593339]
[11]
A Hoang, K Chorath, A Moreira, M Evans, F Burmeister-Morton, and F Burmeister. COVID-19 in 7780 pediatric patients: a systematic review. EClinicalMedicine. 2020;24:100433
. DOI: 10.1016/j.eclinm.2020.100433. [PMID:32766542]
[12]
L Heavey, G Casey, C Kelly, D Kelly, and G McDarby. No evidence of secondary transmission of COVID-19 from children attending school in
Ireland, 2020. Euro Surveill. 2020;25:2000903
. DOI: 10.2807/1560-7917.ES.2020.25.21.2000903. [PMID:32489179]
[13]
C Stein-Zamir, N Abramson, H Shoob, E Libal, M Bitan, and T Cardash. A large COVID-19 outbreak in a high school 10 days after schools’ reopening,
Israel, May 2020. Euro Surveill. 2020;25:2001352
. DOI: 10.2807/1560-7917.ES.2020.25.29.2001352. [PMID:32720636]
[14]
CF Yung, K-q Kam, KD Nadua, CY Chong, NWH Tan, and J Li. Novel coronavirus 2019 transmission risk in educational settings. Clin Infect Dis. 2021;72:1055-8
. DOI: 10.1093/cid/ciaa794. [PMID:32584975]
[15]
A Fontanet
R Grant
L Tondeur
Y Madec
L Grzelak
I Cailleau
SARS-CoV-2 infection in primary schools in northern France: A retrospective cohort
study in an area of high transmission.
MedRxiv. 2020. .DOI: 10.1101/2020.06.25.20140178
[16]
K Macartney, HE Quinn, AJ Pillsbury, A Koirala, L Deng, and N Winkler. Transmission of SARS-CoV-2 in Australian educational settings: a prospective cohort
study. Lancet Child Adolesc Health. 2020;4:807
-16. DOI: 10.1016/S2352-4642(20)30251-0. [PMID:32758454]
[19]
DF Gudbjartsson, A Helgason, H Jonsson, OT Magnusson, P Melsted, and GL Norddahl. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020;382:2302-15
. DOI: 10.1056/NEJMoa2006100. [PMID:32289214]
[21]
P Hallal
F Hartwig
B Horta
GD Victora
M Silveira
C Struchiner
Remarkable variability in SARS-CoV-2 antibodies across Brazilian regions: nationwide
serological household survey in 27 states.
MedRxiv. 2020. DOI: 10.1101/2020.05.30.20117531
[22]
AR Yousaf, LM Duca, V Chu, HE Reses, M Fajans, and EM Rabold. A prospective cohort study in non-hospitalized household contacts with SARS-CoV-2
infection: symptom profiles and symptom change over time. Clin Infect Dis. 2020;Online ahead of printDOI: 10.1093/cid/ciaa1072. [PMID:32719874]
[23]
. Laboratory surveillance for SARS-CoV-2 in India: Performance of testing &
descriptive epidemiology of detected COVID-19, January 22-April 30, 2020. Indian J Med Res. 2020;151:424-
37. DOI: 10.4103/ijmr.IJMR_1896_20. [PMID:32611914]
[26]
M Pollán, B Pérez-Gómez, R Pastor-Barriuso, J Oteo, B Hernán MA, and M Pérez-Olmeda. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based
seroepidemiological study. Lancet. 2020;396:535-44. DOI: 10.1016/S0140-6736(20)31483-5. [PMID:32645347]
[28]
E Bendavid
B Mulaney
N Sood
S Shah
E Ling
R Bromley-Dulfano
Covid-19 antibody seroprevalence in santa clara county, california.
MedRxiv. 2020. DOI: 10.1101/2020.04.14.20062463
[29]
HM Biggs, JB Harris, L Breakwell, FS Dahlgren, GR Abedi, and CM Szablewski. Estimated community seroprevalence of SARS-CoV-2 antibodies—two Georgia
counties, April 28–May 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:965-70. DOI: 10.15585/mmwr.mm6929e2. [PMID:32701941]
[30]
Z Bogogiannidou, A Vontas, K Dadouli, MA Kyritsi, S Soteriades, and DJ Nikoulis. Repeated leftover serosurvey of SARS-CoV-2 IgG antibodies, Greece, March and April
2020. Euro Surveill. 2020;25:2001369
. DOI: 10.2807/1560-7917.ES.2020.25.31.2001369. [PMID:32762796]
[31]
E Lavezzo, E Franchin, C Ciavarella, G Cuomo-Dannenburg, L Barzon, and C Del Vecchio. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’.
Nature. 2020;584:425-9. DOI: 10.1038/s41586-020-2488-1. [PMID:32604404]
[32]
N Menachemi, CT Yiannoutsos, BE Dixon, TJ Duszynski, WF Fadel, and KK Wools-Kaloustian. Population point prevalence of SARS-CoV-2 infection based on a statewide random
sample—Indiana, April 25–29, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:960 DOI: 10.15585/mmwr.mm6929e1. [PMID:32701938]
[33]
N Nawa
J Kuramochi
S Sonoda
Y Yamaoka
Y Nukui
Y Miyazaki
Seroprevalence of SARS-CoV-2 IgG Antibodies in Utsunomiya City, Greater Tokyo, after
first pandemic in 2020 (U-CORONA): a household-and population-based study.
medRxiv. 2020. DOI: 10.1101/2020.07.20.20155945
[34]
G Pagani
F Conti
A Giacomelli
D Bernacchia
R Rondanin
A Prina
Seroprevalence of SARS-CoV-2 IgG significantly varies with age: results from a mass
population screening (SARS-2-SCREEN-CdA).
MedRxiv. 2020. .DOI: 10.1101/2020.06.24.20138875
[36]
M Shakiba, M Nazemipour, A Salari, F Mehrabian, SSH Nazari, and SM Rezvani. Seroprevalence of SARS-CoV-2 in Guilan Province, Iran, April 2020. Emerg Infect Dis. 2021;27:636 DOI: 10.3201/eid2702.201960. [PMID:33349310]
[37]
H Streeck, B Schulte, BM Kümmerer, E Richter, T Höller, and C Fuhrmann. Infection fatality rate of SARS-CoV2 in a super-spreading event in Germany.
Nat Commun. 2020;11:5829 DOI: 10.1038/s41467-020-19509-y. [PMID:33203887]
[38]
S Stringhini, A Wisniak, G Piumatti, AS Azman, SA Lauer, and H Baysson. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland
(SEROCoV-POP): a population-based study. Lancet. 2020;396:313-9. DOI: 10.1016/S0140-6736(20)31304-0. [PMID:32534626]
[39]
M Sutton, P Cieslak, and M Linder. Notes from the Field: Seroprevalence Estimates of SARS-CoV-2 Infection in Convenience
Sample—Oregon, May 11–June 15, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1100
DOI: 10.15585/mmwr.mm6932a4. [PMID:32790658]
[40]
S Weis
A Scherag
M Baier
M Kiehntopf
T Kamradt
S Kolanos
Seroprevalence of SARS-CoV-2 antibodies in an entirely PCR-sampled and quarantined
community after a COVID-19 outbreak-the CoNAN study.
medRxiv. 2020. DOI: 10.1101/2020.07.15.20154112
[41]
S Riley
O Eales
CE Walters
H Wang
KE Ainslie
C Atchinson
REACT-1 round 8 final report: high average prevalence with regional heterogeneity of
trends in SARS-CoV-2 infection in the community in England during January 2021.
medRxiv. 2021. .DOI: 10.1101/2021.01.28.21250606
[42]
S Bignami-van Assche
Y Boujija
D Fisman
J Sandberg
In-person schooling and COVID-19 transmission in Canada’s three largest
cities.
medRxiv. 2021. .DOI: 10.1101/2021.03.21.21254064
[43]
MV Murhekar, T Bhatnagar, S Selvaraju, V Saravanakumar, JWV Thangaraj, and N Shah. SARS-CoV-2 antibody seroprevalence in India, August–September, 2020: findings
from the second nationwide household serosurvey. Lancet Glob Health. 2021;9:e257-66. DOI: 10.1016/S2214-109X(20)30544-1. [PMID:33515512]
[44]
BK Smith, AB Janowski, JE Danis, IB Harvey, H Zhao, and Y-N Dai. Seroprevalence of SARS-CoV-2 Antibodies in Children and Adults in St. Louis,
Missouri, USA. MSphere. 2021;6:e01207-20
. DOI: 10.1128/mSphere.01207-20. [PMID:33536325]
[45]
F González
NA Vielot
M Sciaudone
C Toval-Ruíz
L Premkumar
L Gutierrez
Seroepidemiology of SARS-CoV-2 infections in an urban Nicaraguan population.
medRxiv. 2021. .DOI: 10.1101/2021.02.25.21252447
[46]
HF Gidding, DA Machalek, AJ Hendry, HE Quinn, K Vette, and FH Beard. Seroprevalence of SARS-CoV-2-specific antibodies in Sydney, Australia following the
first epidemic wave in 2020. Med J Aust. 2021;214:179-85
. DOI: 10.5694/mja2.50940. [PMID:33538019]
[47]
KE Wiens
PN Mawien
J Rumunu
D Slater
FK Jones
S Moheed
Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Juba, South Sudan: a
population-based study.
medRxiv. 2021. .DOI: 10.1101/2021.03.08.21253009
[48]
L Chaw
W Koh
S Jamaludin
L Naing
M Alikhan
J Wong
SARS-CoV-2 transmission in different settings: Analysis of cases and close contacts
from the Tablighi cluster in Brunei Darussalam.
medRxiv. 2020. .DOI: 10.1101/2020.05.04.20090043
[49]
H-Y Cheng, S-W Jian, D-P Liu, T-C Ng, W-T Huang, and H-H Lin. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at
different exposure periods before and after symptom onset. JAMA Intern Med. 2020;180:1156-
63. DOI: 10.1001/jamainternmed.2020.2020. [PMID:32356867]
[50]
I Dattner, Y Goldberg, G Katriel, R Yaari, N Gal, and Y Miron. The role of children in the spread of COVID-19: Using household data from Bnei Brak,
Israel, to estimate the relative susceptibility and infectivity of children. PLOS Comput Biol. 2021;17:e1008559
. DOI: 10.1371/journal.pcbi.1008559. [PMID:33571188]
[51]
A James, L Eagle, C Phillips, DS Hedges, C Bodenhamer, and R Brown. High COVID-19 attack rate among attendees at events at a church—Arkansas,
March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:632-635.
[52]
Y Jiang, W Niu, Q Wang, H Zhao, L Meng, and C Zhang. Characteristics of a family cluster of severe acute respiratory syndrome coronavirus
2 in Henan, China. J Infect. 2020;81:e46-8. DOI: 10.1016/j.jinf.2020.04.028. [PMID:32335170]
[53]
. Coronavirus Disease-19: Summary of 2,370 Contact Investigations of the First 30 Cases
in the Republic of Korea. Osong Public Health Res Perspect. 2020;11:81
-4. DOI: 10.24171/j.phrp.2020.11.2.04. [PMID:32257773]
[54]
R Laxminarayan, B Wahl, SR Dudala, K Gopal, S Neelima, and KJ Reddy. Epidemiology and transmission dynamics of COVID-19 in two Indian states.
Science. 2020;370:691-7. DOI: 10.1126/science.abd7672. [PMID:33154136]
[55]
W Li, B Zhang, J Lu, S Liu, Z Chang, and C Peng. Characteristics of household transmission of COVID-19. Clin Infect Dis. 2020;71:1943-6
. DOI: 10.1093/cid/ciaa450. [PMID:32301964]
[56]
T Liu, W Liang, H Zhong, J He, Z Chen, and G He. Risk factors associated with COVID-19 infection: a retrospective cohort study based
on contacts tracing. Emerg Microbes Infect. 2020;9:1546-53. DOI: 10.1080/22221751.2020.1787799. [PMID:32608325]
[57]
AS Lopez, M Hill, J Antezano, D Vilven, T Rutner, and L Bogdanow. Transmission dynamics of COVID-19 outbreaks associated with child care
facilities—Salt Lake City, Utah, April–July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1319
DOI: 10.15585/mmwr.mm6937e3. [PMID:32941418]
[58]
K Mizumoto
R Omori
H Nishiura
Age specificity of cases and attack rate of novel coronavirus disease (COVID-19).
MedRxiv. 2020. .DOI: 10.1101/2020.03.09.20033142
[59]
YJ Park, Y Choe, O Park, S Park, Y Kim, and J Kim. COVID-19 National Emergency Response Center, Epidemiology and Case Management Team.
Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26:2465-
8. DOI: 10.3201/eid2610.201315. [PMID:32673193]
[60]
KM Posfay-Barbe, N Wagner, M Gauthey, D Moussaoui, N Loevy, and A Diana. COVID-19 in children and the dynamics of infection in families. Pediatrics. 2020;146:e20201576
. DOI: 10.1542/peds.2020-1576. [PMID:32457213]
[61]
ES Rosenberg, EM Dufort, DS Blog, EW Hall, D Hoefer, and BP Backenson. COVID-19 testing, epidemic features, hospital outcomes, and household prevalence, New
York State—March 2020. Clin Infect Dis. 2020;71:1953-9
. DOI: 10.1093/cid/ciaa549. [PMID:32382743]
[62]
HH Fore, Q Dongyu, DM Beasley, and TA Ghebreyesus. Child malnutrition and COVID-19: the time to act is now. Lancet. 2020;396:517-8. DOI: 10.1016/S0140-6736(20)31648-2. [PMID:32730742]
[66]
World Health Organization. Addressing violence against children,
women and older people during the COVID-19 pandemic: key actions, 17 June 2020. Geneva: WHO; 2020.
[67]
World Health Organization. Nurturing care for early childhood
development: a framework for helping children survive and thrive to transform health and human
potential. Geneva: WHO; 2018.
[68]
M Komorowski and SK Aberegg. Using applied lung physiology to understand COVID-19 patterns. Br J Anaesth. 2020;125:250-3
. DOI: 10.1016/j.bja.2020.05.019. [PMID:32536444]
[69]
J Xie, N Covassin, Z Fan, P Singh, W Gao, and G Li. Association between hypoxemia and mortality in patients with
COVID-19. Mayo Clin Proc. 2020;95:1138-47
. DOI: 10.1016/j.mayocp.2020.04.006. [PMID:32376101]
[70]
Y Wang, H Tian, L Zhang, M Zhang, D Guo, and W Wu. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use,
disinfection and social distancing: a cohort study in Beijing, China. BMJ Glob Health. 2020;5:e002794
. DOI: 10.1136/bmjgh-2020-002794. [PMID:32467353]
[71]
N Gupta, K Saravu, M Varma, A Pm, S Shetty, and S Umakanth. Transmission of SARS-CoV-2 Infection by Children: A Study of Contacts of Index
Paediatric Cases in India. J Trop Pediatr. 2021;67:fmaa081
. DOI: 10.1093/tropej/fmaa081. [PMID:33280033]
[72]
J Kim, YJ Choe, J Lee, YJ Park, O Park, and MS Han. Role of children in household transmission of COVID-19. Archives of disease in childhood. 2020;DOI: 10.1136/archdischild-2020-319910. [PMID:32769089]
[73]
X-G Kong, J Geng, T Zhang, B Wang, A-Z Wu, and D Xiao. Dynamic profiles of SARS-Cov-2 infection from five Chinese family clusters in the
early stage of the COVID-19 pandemic. Sci Rep. 2020;10:22048 DOI: 10.1038/s41598-020-79035-1. [PMID:33328533]
[74]
L Luo, D Liu, X Liao, X Wu, Q Jing, and J Zheng. Contact settings and risk for transmission in 3410 close contacts of patients with
COVID-19 in Guangzhou, China: a prospective cohort study. Ann Intern Med. 2020;173:879-87
. DOI: 10.7326/M20-2671. [PMID:32790510]
[75]
Q Bi, Y Wu, S Mei, C Ye, X Zou, and Z Zhang. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close
contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;20:911-
9. DOI: 10.1016/S1473-3099(20)30287-5. [PMID:32353347]
[77]
HC Maltezou, R Vorou, K Papadima, A Kossyvakis, N Spanakis, and G Gioula. Transmission dynamics of SARS-CoV-2 within families with children in Greece: A study
of 23 clusters. J Med Virol. 2021;93:1414-20
. DOI: 10.1002/jmv.26394. [PMID:32767703]
[78]
C Atherstone, M Siegel, E Schmitt-Matzen, S Sjoblom, J Jackson, and C Blackmore. SARS-CoV-2 transmission associated with high school wrestling
tournaments—Florida, December 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:141 DOI: 10.15585/mmwr.mm7004e4. [PMID:33507895]
[79]
CM Szablewski, KT Chang, MM Brown, VZ Chu, AR Yousaf, and N Anyalechi. SARS-CoV-2 Transmission and Infection Among Attendees of an Overnight Camp - Georgia,
June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1023
-5. DOI: 10.15585/mmwr.mm6931e1. [PMID:32759921]
[80]
S Desmet, E Ekinci, I Wouters, B Decru, K Beuselinck, and S Malhotra-Kumar. No SARS-CoV-2 carriage observed in children attending daycare centers during the
intial weeks of the epidemic in Belgium. J Med Virol. 2021;93:1828-31
. DOI: 10.1002/jmv.26689. [PMID:33230857]
[81]
JP Torres, C Piñera, V De La Maza, AJ Lagomarcino, D Simian, and B Torres. SARS-CoV-2 antibody prevalence in blood in a large school community subject to a
Covid-19 outbreak: a cross-sectional study. Clinical Infectious Diseases. 2020;[PMID:32649743]
[82]
T Dub
E Erra
L Hagberg
E Sarvikivi
C Virta
A Jarvinen
Transmission of SARS-CoV-2 following exposure in school settings: experience from two
Helsinki area exposure incidents.
medRxiv. 2020. .DOI: 10.1101/2020.07.20.20156018
[83]
A Fontanet
L Tondeur
Y Madec
R Grant
C Besombes
N Jolly
Cluster of COVID-19 in northern France: A retrospective closed cohort study.
medRxiv. 2020. .DOI: 10.1101/2020.04.18.20071134
[84]
JP Armann
M Unrath
C Kirsten
C Lück
A Dalpke
R Berner
Anti-SARS-CoV-2 IgG antibodies in adolescent students and their teachers in Saxony,
Germany (SchoolCoviDD19): very low seropraevalence and transmission rates.
2020. .DOI: 10.1101/2020.07.16.20155143
[85]
NE Brown, J Bryant-Genevier, U Bandy, CA Browning, AL Berns, and M Dott. Antibody responses after classroom exposure to teacher with coronavirus disease,
March 2020. Emerg Infect Dis. 2020;26:2263 DOI: 10.3201/eid2609.201802. [PMID:32597750]
[86]
LL Blaisdell, W Cohn, JR Pavell, DS Rubin, and JE Vergales. Preventing and mitigating SARS-CoV-2 transmission—four overnight camps, Maine,
June–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1216
-20. DOI: 10.15585/mmwr.mm6935e1. [PMID:32881850]
[87]
R Link-Gelles, AL DellaGrotta, C Molina, A Clyne, K Campagna, and TM Lanzieri. Limited secondary transmission of SARS-CoV-2 in child care programs—Rhode
Island, June 1–July 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1170
-2. DOI: 10.15585/mmwr.mm6934e2. [PMID:32853185]
[88]
Surveillance NCIRS. COVID-19 in schools and early childhood
education and care services-The term 1 experience in NSW. National Centre for Immunisazion Research
and Surveillance (NCIRS); July 31, 2020 2020. Available:
https://www.ncirs.org.au/covid-19-in-schools. Accessed: 22 February 2021.
[89]
Surveillance NCIRS. COVID-19 in schools and early childhood
education and care services-The term 2 experience in NSW. National Centre for Immunisazion Research
and Surveillance (NCIRS); July 31, 2020 2020. Available:
https://www.ncirs.org.au/covid-19-in-schools. Accessed: 22 February 2021.
[90]
Surveillance NCIRS. COVID-19 in schools and early childhood
education and care services-The term 3 experience in NSW. National Centre for Immunisazion Research
and Surveillance (NCIRS); July 31, 2020 2020. Available:
https://www.ncirs.org.au/covid-19-in-schools. Accessed: 22 February 2021.
[91]
SA Ismail, V Saliba, JL Bernal, ME Ramsay, and SN Ladhani. SARS-CoV-2 infection and transmission in educational settings: a prospective,
cross-sectional analysis of infection clusters and outbreaks in England. Lancet Infect Dis. 2021;21:344-
53. DOI: 10.1016/S1473-3099(20)30882-3. [PMID:33306981]
[92]
LT Brandal, TS Ofitserova, H Meijerink, R Rykkvin, HM Lund, and O Hungnes. Minimal transmission of SARS-CoV-2 from paediatric COVID-19 cases in primary schools,
Norway, August to November 2020. Euro Surveill. 2021;26:2002011
. DOI: 10.2807/1560-7917.ES.2020.26.1.2002011. [PMID:33413743]
[93]
E Larosa, O Djuric, M Cassinadri, S Cilloni, E Bisaccia, and M Vicentini. Secondary transmission of COVID-19 in preschool and school settings in northern Italy
after their reopening in September 2020: a population-based study. Euro Surveill. 2020;25:2001911
. DOI: 10.2807/1560-7917.ES.2020.25.49.2001911. [PMID:33303065]
[94]
Y Yoon
K-R Kim
H Park
young Kim S, Kim Y-J. Stepwise school opening online and off-line and an impact on
the epidemiology of COVID-19 in the pediatric population.
medRxiv. 2020. .DOI: 10.1101/2020.08.03.20165589
[95]
M Okarska-Napierała, J Mańdziuk, and E Kuchar. SARS-CoV-2 Cluster in Nursery, Poland. Emerg Infect Dis. 2021;27:317 DOI: 10.3201/eid2701.203849. [PMID:33035153]
[96]
S Kriemler
A Ulyte
P Ammann
GP Peralta
C Berger
MA Puhan
Surveillance of acute SARS-CoV-2 infections in school children and point-prevalence
during a time of high community transmission in Switzerland.
medRxiv. 2021. .DOI: 10.1101/2020.12.24.20248558
[97]
A Ulyte, T Radtke, IA Abela, SR Haile, C Berger, and M Huber. Clustering and longitudinal change in SARS-CoV-2 seroprevalence in school-children:
prospective cohort study of 55 schools in Switzerland. BMJ. 2021;372:n616 DOI: 10.1136/bmj.n616. [PMID:33731327]
[99]
SN Ladhani, F Baawuah, J Beckmann, IO Okike, S Ahmad, and J Garstang. SARS-CoV-2 infection and transmission in primary schools in England in
June–December, 2020 (sKIDs): an active, prospective surveillance study. Lancet Child Adolesc Health. 2021;5:417
-27. DOI: 10.1016/S2352-4642(21)00061-4. [PMID:33740430]
[100]
CM Szablewski, KT Chang, CJ McDaniel, VT Chu, AR Yousaf, and NG Schwartz. SARS-CoV-2 transmission dynamics in a sleep-away camp. Pediatrics. 2021;147:e2020046524
. DOI: 10.1542/peds.2020-046524. [PMID:33504612]
[101]
E Lachassinne, L de Pontual, M Caseris, M Lorrot, C Guilluy, and A Naud. SARS-CoV-2 transmission among children and staff in daycare centres during a
nationwide lockdown in France: a cross-sectional, multicentre, seroprevalence study.
Lancet Child Adolesc Health. 2021;5:256
-64. DOI: 10.1016/S2352-4642(21)00024-9. [PMID:33571450]
[102]
U Heudorf, K Steul, A Walczok, and R Gottschalk. Children and COVID-19-Data from mandatory reporting and results of contact person
testing in daycare centers and schools in Frankfurt am Main, Germany. Monatsschrift Kinderheilkunde. 2021;2021:1
-11. [PMID:33678906]
[103]
KG Volpp, BH Kraut, S Ghosh, and J Neatherlin. Minimal SARS-CoV-2 Transmission After Implementation of a Comprehensive Mitigation
Strategy at a School—New Jersey, August 20–November 27, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:377 DOI: 10.15585/mmwr.mm7011a2. [PMID:33735161]
[104]
IW Pray, SN Gibbons-Burgener, AZ Rosenberg, D Cole, S Borenstein, and A Bateman. COVID-19 Outbreak at an Overnight Summer School Retreat—Wisconsin,
July–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1600
DOI: 10.15585/mmwr.mm6943a4. [PMID:33119558]
[105]
RM Viner, OT Mytton, C Bonell, G Melendez-Torres, J Ward, and L Hudson. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with
adults: a systematic review and meta-analysis. JAMA Pediatr. 2021;175:143-56
. DOI: 10.1001/jamapediatrics.2020.4573. [PMID:32975552]
[106]
J Merckx, JA Labrecque, and JS Kaufman. Transmission of SARS-CoV-2 by children. Dtsch Arztebl Int. 2020;117:553 [PMID:32705983]
[107]
FS Lu
AT Nguyen
NB Link
M Lipsitch
M Santillana
Estimating the early outbreak cumulative incidence of COVID-19 in the United States:
three complementary approaches.
medRxiv. 2020.
[108]
R Castagnoli, M Votto, A Licari, I Brambilla, R Bruno, and S Perlini. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children
and adolescents: a systematic review. JAMA Pediatr. 2020;174:882-9
. DOI: 10.1001/jamapediatrics.2020.1467. [PMID:32320004]
[109]
E Goldstein, M Lipsitch, and M Cevik. On the Effect of Age on the Transmission of SARS-CoV-2 in Households, Schools, and
the Community. J Infect Dis. 2021;223:362-9
. DOI: 10.1093/infdis/jiaa691. [PMID:33119738]
[110]
W Xu, X Li, M Dozier, Y He, A Kirolos, and Z Lang. What is the evidence for transmission of COVID-19 by children in schools? A living
systematic review. J Glob Health. 2020;10:021104
. DOI: 10.7189/jogh.10.021104. [PMID:33437465]
[111]
TK Tsang, VJ Fang, K-H Chan, DK Ip, GM Leung, and JM Peiris. Individual correlates of infectivity of influenza A virus infections in households.
PLoS One. 2016;11:e0154418
. DOI: 10.1371/journal.pone.0154418. [PMID:27153194]
[112]
M Paunio, H Peltola, M Valle, I Davidkin, M Virtanen, and OP Heinonen. Explosive school-based measles outbreak: intense exposure may have resulted in high
risk, even among revaccinees. Am J Epidemiol. 1998;148:1103-
10. DOI: 10.1093/oxfordjournals.aje.a009588. [PMID:9850133]
[113]
FP Lyngse
K Mølbak
K Træholt Frank
C Nielsen
RL Skov
CT Kirkeby
Association between SARS-CoV-2 transmission risk, viral load, and age: a nationwide
study in Danish households.
medRxiv. .DOI: 10.1101/2021.02.28.21252608
[114]
O Byambasuren, M Cardona, K Bell, J Clark, M-L McLaws, and P Glasziou. Estimating the extent of asymptomatic COVID-19 and its potential for community
transmission: systematic review and meta-analysis. JAMMI. 2020;5:223-34. DOI: 10.3138/jammi-2020-0030
[115]
CA Rostad, S Kamidani, and EJ Anderson. Implications of SARS-CoV-2 Viral Load in Children: Getting Back to School and Normal.
JAMA Pediatr. 2021;Epub ahead of printDOI: 10.1001/jamapediatrics.2021.2022. [PMID:34115097]
[116]
KO Zimmerman, IC Akinboyo, MA Brookhart, AE Boutzoukas, K McGann, and MJ Smith. Incidence and secondary transmission of SARS-CoV-2 infections in schools.
Pediatrics. 2021;Epub ahead of printDOI:
10.1542/peds.2020-048090. [PMID:33419869]
[117]
KA Auger, SS Shah, T Richardson, D Hartley, M Hall, and A Warniment. Association between statewide school closure and COVID-19 incidence and mortality in
the US. JAMA. 2020;324:859-70. DOI: 10.1001/jama.2020.14348. [PMID:32745200]
[118]
J Vlachos, E Hertegård, and HB Svaleryd. The effects of school closures on SARS-CoV-2 among parents and teachers.
Proc Natl Acad Sci U S A. 2021;118:
e2020834118. DOI: 10.1073/pnas.2020834118. [PMID:33574041]
[119]
JA Gold. Clusters of SARS-CoV-2 infection among elementary school educators and students in
one school district—Georgia, December 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:289-92. DOI: 10.15585/mmwr.mm7008e4. [PMID:33630823]
[120]
N Ziauddeen, K Woods-Townsend, S Saxena, R Gilbert, and NA Alwan. Schools and COVID-19: reopening Pandora’s Box? Public Health in Practice. 2020;1:100039. DOI:
10.1016/j.puhip.2020.100039
[121]
W Van Lancker and Z Parolin. COVID-19, school closures, and child poverty: a social crisis in the making.
Lancet Public Health. 2020;5:e243-4. DOI: 10.1016/S2468-2667(20)30084-0. [PMID:32275858]
[122]
X Xie, Q Xue, Y Zhou, K Zhu, Q Liu, and J Zhang. Mental health status among children in home confinement during the coronavirus
disease 2019 outbreak in Hubei Province, China. JAMA Pediatr. 2020;174:898-900
. DOI: 10.1001/jamapediatrics.2020.1619. [PMID:32329784]
[123]
G Wang, Y Zhang, J Zhao, J Zhang, and F Jiang. Mitigate the effects of home confinement on children during the COVID-19 outbreak.
Lancet. 2020;395:945-47. DOI: 10.1016/S0140-6736(20)30547-X. [PMID:32145186]
[124]
CG Dunn, E Kenney, SE Fleischhacker, and SN Bleich. Feeding low-income children during the Covid-19 pandemic. N Engl J Med. 2020;382:e40
. DOI: 10.1056/NEJMp2005638. [PMID:32227759]
[125]
Hallgarten J. Evidence on efforts to mitigate the negative
educational impact of past disease outbreaks. 2020 Report 793. Reading, UK: Education Development
Trust. 2020.
[126]
Government of Sierra Leone. National Ebola Recovery Strategy for
Sierra Leone: 2015-2017. Freetown: Government of Sierra Leone; 2015.
[128]
ME Eltahir. E-learning in developing countries: Is it a panacea? A case study of Sudan.
IEEE Access. 2019;7:97784-92
. DOI: 10.1109/ACCESS.2019.2930411
[129]
M Levinson, M Cevik, and M Lipsitch. Reopening primary schools during the pandemic. N Engl J Med. 2020;383:981-5
. DOI: 10.1056/NEJMms2024920. [PMID:32726550]
[130]
F Coronado, S Blough, D Bergeron, K Proia, E Sauber-Schatz, and M Beltran. Implementing mitigation strategies in early care and education settings for
prevention of SARS-CoV-2 transmission—eight states, September–October 2020.
MMWR Morb Mortal Wkly Rep. 2020;69:1868
DOI: 10.15585/mmwr.mm6949e3. [PMID:33301431]
[134]
L Jiang, K Tang, M Levin, O Irfan, SK Morris, and K Wilson. COVID-19 and multisystem inflammatory syndrome in children and adolescents.
Lancet Infect Dis. 2020;20:e276-e288. DOI: 10.1016/S1473-3099(20)30651-4. [PMID:32818434]
|
|
 |



















